Designing and All-In-One Filter
Published on by Water Network Research, Official research team of The Water Network in Academic
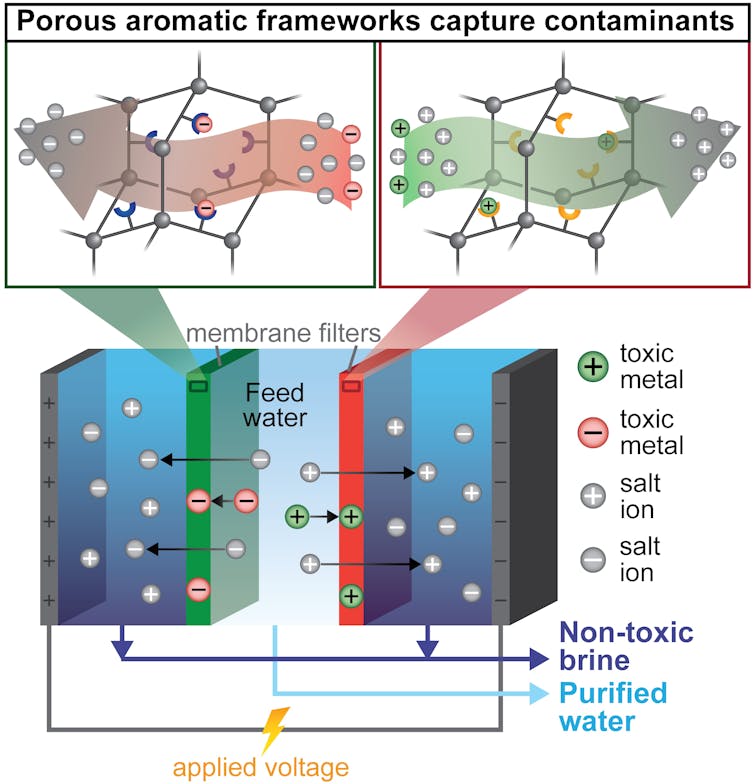
To build a single filter that could both capture metals and remove salt, my colleagues and I first needed a material that could remove many different contaminants – mostly heavy metals – from water. To do this, we turned to tiny, absorbent particles called porous aromatic frameworks. These particles are designed to selectively capture individual contaminants. For example, one type of absorbent particle can catch only mercury. Other types specifically remove only copper, iron, or boron. I then embedded these four different types of particles into thin plastic membranes, essentially creating custom filters that would capture contaminants according to the type of particle I put in the membrane.
A colleague and I then placed these membrane filters into an electrodialysis water purifier. Electrodialysis is a method that uses electricity to pull salts and toxins out of water, across a membrane and into a separate waste stream. This waste – often called brine – can become toxic and expensive to dispose in existing desalination processes.
This new approach to desalination – called ion-capture electrodialysis – uses thin membranes and electricity to capture toxic metals as they are pulled from water along with salts. Ada Uliana, CC BY-ND
In my team’s modified process, called ion-capture elecrodialysis, our hope was that the membranes packed with the tiny metal-absorbing particles would capture toxic metals instead of allowing them to move into the brine. This would achieve three benefits at the same time in an energy-efficient manner: Salts and metals would be removed from the water; the toxic metals would be captured in a small, easily disposable membrane – or even potentially be reused; and the salty waste stream would be nontoxic.
How effective is ion-capture electrodialysis?
Once our team had successfully made these membranes, we needed to test them. The first test I ran used membrane filters embedded with mercury-capturing absorbents to purify water from three sources that contained both mercury and salts: groundwater, brackish water and industrial wastewater. To our team’s excitement, the membranes captured all the mercury in every test. Additionally, the membranes were also great at getting rid of salt – over 97% was removed from the dirty water. After just one pass through our new electrodialysis machine, the water was perfectly drinkable. Importantly, further experiments showed that no mercury can pass through the filter until nearly all the absorbent particles in the filter are used up.
My colleagues and I then needed to see whether our ion-capture electrodialysis process would work on other common harmful metals. I tested three membrane filters that contained absorbents for copper, iron or boron. Every filter was a success. Each filter captured all of the target contaminants without any detectable amount passing into the brine, while simultaneously removing over 96% of salts from the water, purifying the water to usable conditions.
Remaining challenges
Our results show that our new water purification method can selectively capture many common contaminants while also removing salt from water. But there are still other technological challenges to figure out.
Taxonomy
- Filters
- Filtration Solutions
- Filtration
- Membrane Filtration
- Water filtration, purifiers, nano filtration