NEWS NOTES ON SUSTAINABLE WATER RESOURCESOcean Acidificationhttps://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification...
Published on by Tim Smith, Employee at Retiree & P/T Consultant
Ocean Acidification
https://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification
In the 200-plus years since the industrial revolution began, the concentration of carbon dioxide (CO2) in the atmosphere has increased due to human actions. During this time, the pH of surface ocean waters has fallen by 0.1 pH units. This might not sound like much, but the pH scale is logarithmic, so this change represents approximately a 30 percent increase in acidity.
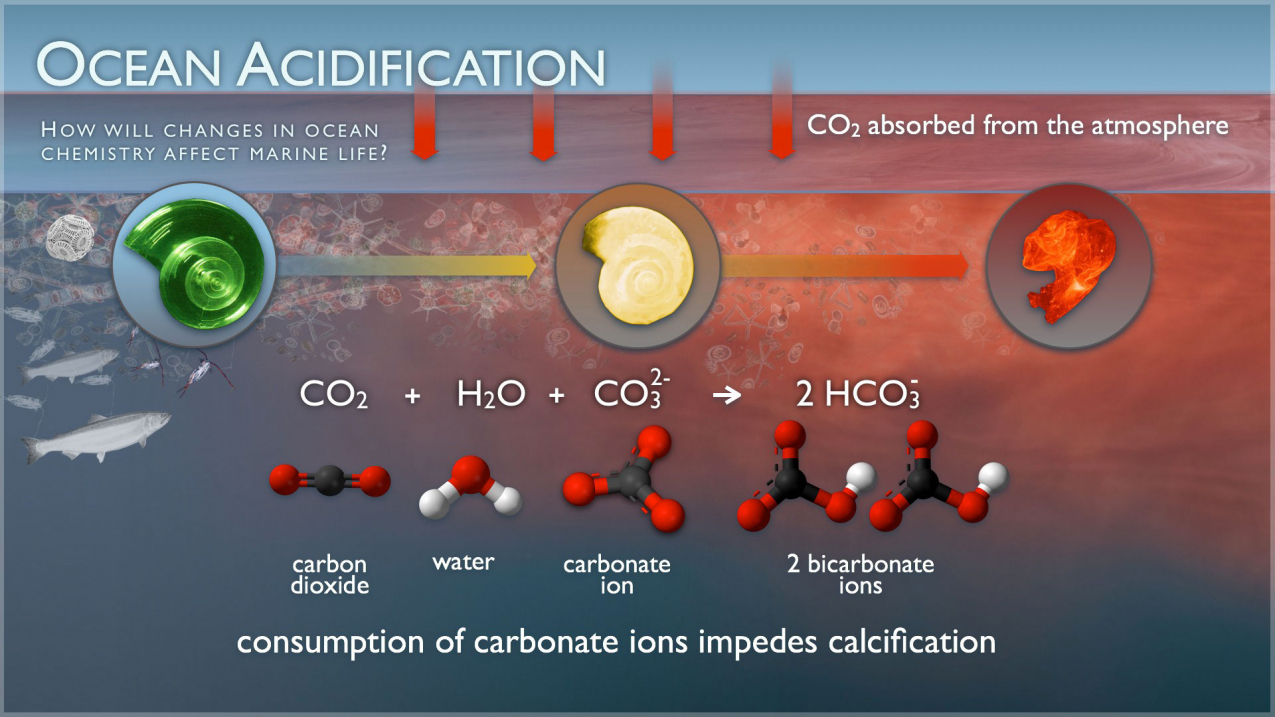
The ocean absorbs about 30% of the carbon dioxide (CO2) that is released in the atmosphere. As levels of atmospheric CO2 increase from human activity such as burning fossil fuels (e.g., car emissions) and changing land use (e.g., deforestation), the amount of carbon dioxide absorbed by the ocean also increases. When CO2 is absorbed by seawater, a series of chemical reactions occur resulting in the increased concentration of hydrogen ions. This process has far reaching implications for the ocean and the creatures that live there.
Carbon dioxide, which is naturally in the atmosphere, dissolves into seawater. Water and carbon dioxide combine to form carbonic acid (H2CO3), a weak acid that breaks (or “dissociates”) into hydrogen ions (H+) and bicarbonate ions (HCO3-).
Because of human-driven increased levels of carbon dioxide in the atmosphere, there is more CO2 dissolving into the ocean. The ocean’s average pH is now around 8.1 offsite link, which is basic (or alkaline), but as the ocean continues to absorb more CO2, the pH decreases and the ocean becomes more acidic.
Ocean acidification is one aspect of global climate change. Anything we do to mitigate climate change today will benefit the future of the ocean as well. Over the last decade, there has been much focus in the ocean science community on studying the potential impacts of ocean acidification. NOAA's Ocean Acidification Program serves to build relationships between scientists, resource managers, policy makers, and the public in order to research and monitor the effects of changing ocean chemistry on economically and ecologically important ecosystems such as fisheries and coral reefs.
Because sustained efforts to monitor ocean acidification worldwide are only beginning, it is currently impossible to predict exactly how ocean acidification impacts will cascade throughout the marine food web and affect the overall structure of marine ecosystems. With the pace of ocean acidification accelerating, scientists, resource managers, and policymakers recognize the urgent need to strengthen the science as a basis for sound decision making and action.
NOTE: More information on Sustainable Water Resources is available at https://sites.google.com/site/sustainablewaterresources/