Defense Mechanism Employed by Algae Inhibit Marine Fouling
Published on by Water Network Research, Official research team of The Water Network in Academic
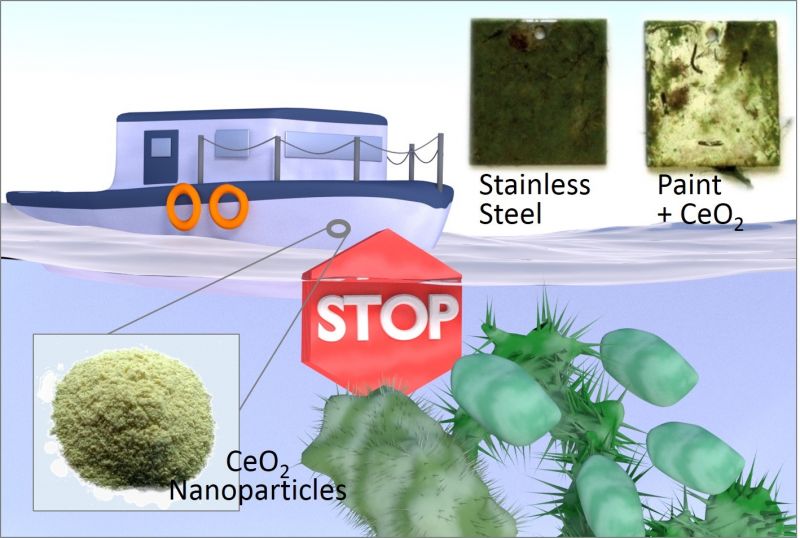
Chemists at Johannes Gutenberg University Mainz (JGU) have developed a method that reliably hinders hazardous seawater fouling and is effective, affordable, and easy on the environment.
Like the natural enzyme vanadium bromoperoxidase cerium dioxide nanoparticles act as a catalyst for the formation of hypobromous acid from bromide ions (contained in sea water) and small amounts of hydrogen peroxide that are formed upon exposure to sun light yielding reduced biofilm formation. Credit: ill./©: Tremel research group, JGU
Fouling can occur, for example, as the result of the growth of bacteria, algae, or mollusks in harbor facilities, on boat hulls, and aquaculture netting. The resultant damage and consequential costs can be significant. It is estimated that these are equivalent to 200 billion dollars annually in the shipping industry alone. Protective coatings applied to vessels usually contain copper-based biocides.
These have the disadvantage that they harm the environment while resistance to them can also develop. In order to find an alternative, the Mainz-based research team of Professor Wolfgang Tremel decided to simulate a defense mechanism employed by algae and established that cerium dioxide nanoparticles can effectively prevent fouling. This discovery could contribute towards the production of new protective coatings that are much less environmentally harmful than the hull coatings in use to date.
Marine algae utilize secondary metabolic products in order to provide themselves with a form of chemical defense against micro-organisms and predators. These halogenated secondary metabolites specifically prevent bacterial biofilms, other algae, and even barnacles becoming attached to and developing on larger formations of algae, sponges, and other creatures.
 Halogenated compounds produced by the red seaweed Delisea pulchra, for instance, inhibit bacterial fouling but are neither toxic nor growth-retarding.
Halogenated compounds produced by the red seaweed Delisea pulchra, for instance, inhibit bacterial fouling but are neither toxic nor growth-retarding.
Instead, they scupper what is known as quorum sensing, i.e, a system used by bacteria to communicate with the help of messenger substances that results in the formation of biofilms.
The structures of the halogenated compounds synthesized by seaweeds are similar to those of these substances so that they cause a blockade of the bacterial receptors and suppress the switchover of bacterial gene regulation to biofilm formation.
This form of interference with bacterial gene regulation is also of pharmaceutical interest as it is known that pathogenic bacteria can protect themselves against attack by the immune system and the effect of antibiotics by forming biofilms, for instance on the epithelium of the respiratory system.
Biofilms are around virtually everywhere. They are present in drinking water pipes and clarification plants, in ground water, water filtration and cooling systems, on practically all surfaces such as food packaging, door handles, push buttons, keyboards, and other elements made of plastic, and, when it comes to medicine, they also develop in catheter tubes.
The main problem in connection with combating these using biocides and antibiotics is the risk of the development of resistance.
Read more: Phys.org
Media
Taxonomy
- Technology
- Filtration
- Algae
- Marine
- Oceanographic Survey
- Environmental Impact
- Marine Technologies