Filtration methods remove harmful 'forever chemicals' from drinking water
Published on by Water Network Research, Official research team of The Water Network in Technology
by Danielle Underferth, Johns Hopkins University
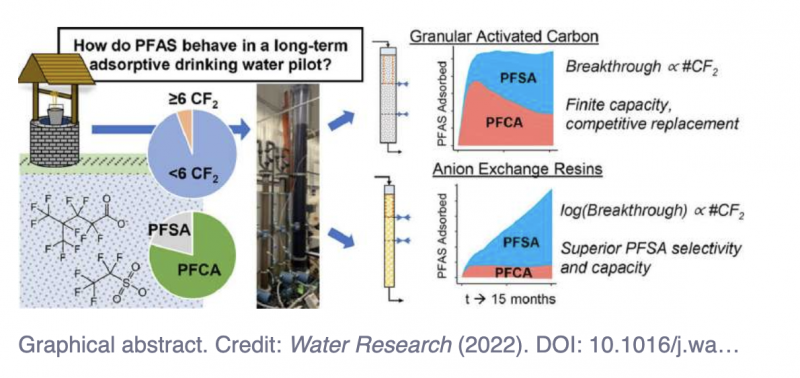
Graphical abstract. Credit: Water Research (2022). DOI: 10.1016/j.watres.2022.119198
A team of Johns Hopkins engineers has discovered a method to filter out a class of harmful industrial "forever chemicals" commonly found in the country's drinking water.
Known as forever chemicals because they last thousands of years, per- and polyfluoroalkyl substances, commonly known as PFAS, are a group of synthetic organic contaminants used in a variety of industrial processes and consumer products, including non-stick cookware coatings, food wrappers, water-resistant clothing, and furniture textiles. When these products are used, discarded, or improperly dumped, PFAS leach into water systems. Once there, they are incredibly difficult to treat because of unique chemical properties imparted by carbon-fluorine bonds—some of the strongest in chemistry.
The precise amount of PFAS in the country's drinking water is unknown, but experts say that it ranges from widespread to almost ubiquitous.
Long-term, low-concentration exposure to PFAS can hamper the immune system, interfere with hormones, and reduce the effectiveness of vaccines. It can also cause low birth weight and high cholesterol. Those who are exposed to high doses of PFAS at work or because they live near a dumping site are at increased risk for kidney cancer, liver damage, testicular cancer, and thyroid disease.
The team compared two of the most promising types of separation media—granular-activated carbon and anion exchange resins—to remove PFAS. They systematically evaluated how well each of these worked for different types of PFAS in pilot municipal water treatment plants for 15 months, concluding that the resins were more effective in removing most of the tested PFAS.
"You can think of the treatments as being large-scale versions of the filters people use at home to purify their own drinking water. Water is continuously fed from the top, and we measure the amount of time before we start to detect PFAS coming out of the end of the filter," says Steven Chow, research associate in the Department of Environmental Health and Engineering at Johns Hopkins and first author of the study. The results appeared in Water Research.
The researchers say that the study is promising because it provides one of the most in-depth laboratory analyses of a large-scaled engineered PFAS treatment system to date. Normally, laboratory research has limitations in practical applicability because it's conducted on a small-scale over limited time. And many large-scale engineered systems run by drinking water utilities do not have the luxury of in-depth analysis of their systems beyond regulatory requirements, the engineers say.
"Our collaboration between academia, industry, and municipal partners allowed the team to answer fundamental questions on how these separation media work while demonstrating their effectiveness under real-world conditions," Chow says.
While the technical outlook for filtering PFAS from drinking water is promising, the researchers warn that there are caveats. Building and operating such systems can cost millions of dollars, which is in addition to the cost of existing water treatment operations. Once the PFAS are removed, the contaminants and the filter used to collect them must be properly handled and disposed of, and because PFAS molecules are so strong, an incredible amount of energy is required to destroy them.