High-efficient and Low-cost Catalyst for Water Electrolysis
Published on by Water Network Research, Official research team of The Water Network in Academic
A team of researchers in Korea has successfully developed an alternative and cheap anode material for excellent and ultra-stable alkaline water electrolysis.
A research team led by Professor Sangaraju Shanmugam of Energy Science and Engineering at DGIST has developed highly efficient and ultra-durable core-shell nanostructured electrocatalyst and successfully replaced the precious anode in water electrolysis, through the collaboration with the research group of Pacific Northwest National Laboratory (PNNL), USA.
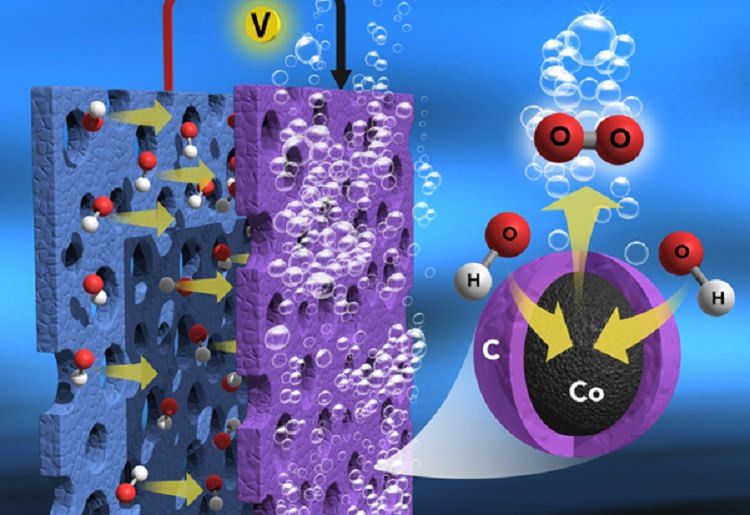
Schematic representation of water splitting and possible electron movements through the nanocarbon layers in NC-trapped single cobalt atom. Copyright : Daegu Gyeongbuk Institute of Science and Technology (DGIST)
The replacement of conventional fuels with renewable energy resources is a suitable approach to achieving an eco-friendly environment and decreasing future energy demands. To these ends, electrochemical energy generation or conversion in renewable energy devices, which depends on anode and cathode reactions, has received much attention.
In electrocatalytic water splitting, oxygen gas generates in the anode due to the oxygen evolution reaction (OER), and it is a slow electrochemical reaction as compared with the hydrogen evolution reaction (HER). Thus, a suitable electrocatalyst is needed for promising and stable electrocatalytic water splitting.
Development of efficient, durable, low-cost OER electrocatalysts is a great challenge and paid more attention to the renewable energy devices of the water electrolyzer. Until now, the Ruthenium and Iridium oxides are considered as state-of-the-art electrocatalysts in OER, but the lack of stability limit their use in large-scale water splitting and hinder widespread commercialization.
Hence, Professor Shanmugam’s and PNNL teams has focused on developing an alternative, low-cost, non-noble metal electrocatalyst to replace the noble metal anode electrode in efficient water splitting. Carbon-supported metal has been considered as an efficient electrocatalytic material for the enhanced OER in water splitting.
So far, most of the developed electrocatalysts have featured higher carbon content and less metal active specious content. The higher carbon amount mired the real metal active sites and thus resulted in a faster carbon corrosion conditions. This further led to lower electrocatalytic activity, stability and large-scale water splitting processes (scalability).
In the study, the researchers found that a large amount of inorganic cobalt metal ions bridged by organic ligands in the Prussian blue analog were shown to be a suitable precursor for developing efficient and ultra-stable, metal-rich, nitrogen-doped graphitic nanocarbon-encapsulated core-shell electrocatalysts for the sluggish OER (anode) in water splitting.
When heated (600–900°C) in an inert atmosphere, the cobalt metal ions and organic ligands in the salt are transformed into cobalt metal and nitrogen-doped graphitic thin carbon layers, respectively, which form the thin carbon layer, encapsulated metallic, cobalt core-shell nanostructures (Core-Shell Co@NC).
The thin carbon layers have a strong interaction with cobalt metal, which can promote less carbon corrosion, excellent electron movement, and more cobalt metal exposure to the reaction medium, including the formation of nanosized morphology without particle aggregation.
Accordingly, the combined effect of carbon and cobalt metal in the electrodes achieves the more efficient electrocatalytic activity of the OER than that of the precious metal electrodes to allow efficient water splitting. Therefore, the non-noble metal-rich electrode is an alternative, active, stable, and less expensive OER anode for cost-effective H2 gas production in commercial-scale water electrolysis.
"Anticipate this to be a unique approach to developing metal-rich, reduced-carbon composite nanostructures that have enhanced metal active sites, which feature thin carbon layer protection and ultra-fast electron movement in the catalyst surface, that will enhance the electrochemical activity and stability of electrocatalysts,” says Professor Shanmugam. “We will carry out the follow-up studies that can be used to understand the real OER mechanism on the active species in the presence of nanocarbon coating".
This research outcome was published in the online edition of Advanced Energy Materials on 11th of January 2018, a reputed international journal in the field emerging materials.
Read full article and an interview with Professor Sangaraju Shanmugam: Asia Research News
Media
Taxonomy
- Treatment
- Electrolosis
- Treatment Methods
- water treatment