Optimizing Carbon Nanotube Electrodes
Published on by Water Network Research, Official research team of The Water Network in Academic
New model measures characteristics of carbon nanotube structures for energy storage and water desalination applications.
By Nancy W. Stauffer | MIT Energy Initiative
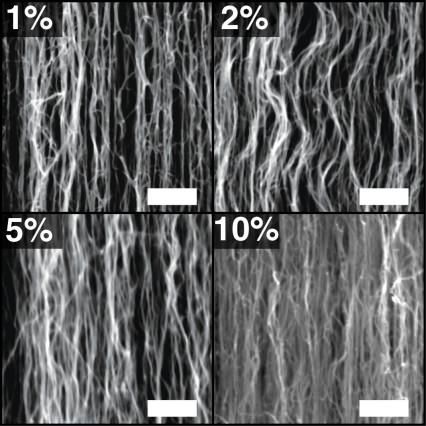
Scanning electron microscope (SEM) images of CNT coatings. These images show samples of CNT forests at varying volume fractions. At top left is the as-fabricated sample with a volume fraction of 1 percent (meaning 1 percent of the total volume is occupied by nanotubes). The other images show denser samples with volume fractions of 2 percent, 5 percent, and 10 percent. The scale bar on each image is 500 nanometers. Courtesy of the researchers, via MIT
Using electrodes made of carbon nanotubes (CNTs) can significantly improve the performance of devices ranging from capacitors and batteries to water desalination systems. But figuring out the physical characteristics of vertically aligned CNT arrays that yield the most benefit has been difficult.
Now an MIT team has developed a method that can help. By combining simple benchtop experiments with a model describing porous materials, the researchers have found they can quantify the morphology of a CNT sample, without destroying it in the process.
In a series of tests, the researchers confirmed that their adapted model can reproduce key measurements taken on CNT samples under varying conditions. They’re now using their approach to determine detailed parameters of their samples — including the spacing between the nanotubes — and to optimize the design of CNT electrodes for a device that rapidly desalinates brackish water.
A common challenge in developing energy storage devices and desalination systems is finding a way to transfer electrically charged particles onto a surface and store them there temporarily. In a capacitor, for example, ions in an electrolyte must be deposited as the device is being charged and later released when electricity is being delivered. During desalination, dissolved salt must be captured and held until the cleaned water has been withdrawn.
One way to achieve those goals is by immersing electrodes into the electrolyte or the saltwater and then imposing a voltage on the system. The electric field that's created causes the charged particles to cling to the electrode surfaces. When the voltage is cut, the particles immediately let go.
"Whether salt or other charged particles, it’s all about adsorption and desorption," says Heena Mutha PhD '17, a senior member of technical staff at the Charles Stark Draper Laboratory. "So the electrodes in your device should have lots of surface area as well as open pathways that allow the electrolyte or saltwater carrying the particles to travel in and out easily."
One way to increase the surface area is by using CNTs. In a conventional porous material, such as activated charcoal, interior pores provide extensive surface area, but they're irregular in size and shape, so accessing them can be difficult. In contrast, a CNT "forest" is made up of aligned pillars that provide the needed surfaces and straight pathways, so the electrolyte or saltwater can easily reach them.
However, optimizing the design of CNT electrodes for use in devices has proven tricky. Experimental evidence suggests that the morphology of the material — in particular, how the CNTs are spaced out — has a direct impact on device performance.
Increasing the carbon concentration when fabricating CNT electrodes produces a more tightly packed forest and more abundant surface area. But at a certain density, performance starts to decline, perhaps because the pillars are too close together for the electrolyte or saltwater to pass through easily.
Read full article: MIT
Media
Taxonomy
- Technology
- Nanotechnology
- Filtration
- Desalination
- Sea Water Desalinisation
- Sustainable Desalination
- Desalination
- Nanotubes