New Material Separates Oil and Water For Environmental Remediation and Wastewater Treatment
Published on by Water Network Research, Official research team of The Water Network in Technology
A Tufts research team develops material to separate oil and water for environmental remediation and wastewater treatment.
By Mike Silver, Tufts Now
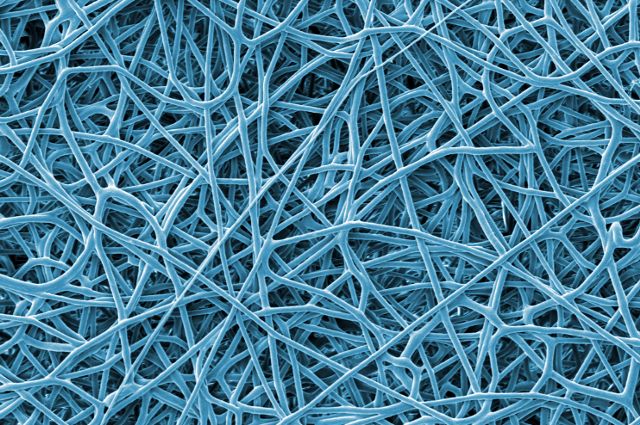
“Filtration is a simple, energy-efficient water treatment method, and might be an effective way to clean up oil spills,” said Ayse Asatekin. Here, an image of the new fiber membrane showing its random porous structure. (Image by NelakaGovinna for Tufts Now)
It’s a popular phrase used to describe people, things, and ideas that just don’t mix—“like oil and water.” Except it’s not entirely true. Oil and water can mix, and can be very difficult to completely separate when brought together. Think of environmental oil spills or wastewater treatment, and you quickly realize that separating out unwanted oil to restore water to a natural or pure state can be a monumental task.
In a research paper published on March 22—the United Nations-designated World Water Day—engineers and physicists from Tufts describe how they devised a low-cost membrane capable of rapidly filtering oil from water and oil mixtures without the membrane getting fouled.
The advance in material science could be a game changer in the battle against pollution. It’s well known that oil-contaminated water can have long-term harmful effects on wildlife and the environment. Current strategies to mitigate that harm include burning the oil in place or using mechanical devices, such as booms, skimmers, or absorbent material, to help clean up the mess. In practice these methods are expensive and not very effective, particularly for cleaning up large oil spills.
“Filtration is a simple, energy-efficient water treatment method, and might be an effective way to clean up these oil spills,” said Ayse Asatekin, an assistant professor in the Tufts School of Engineering and corresponding author of the study, published in the journal ACS Applied Polymer Materials. “A separation membrane is relatively inexpensive and reusable, and the clean-up technology covers a small footprint. Through our collaboration, we developed a novel filter material that can do the separation and keep up a high flow rate without getting fouled by accumulating oil.”
Fortunately, nature provides some examples of materials that interact very differently with water and oil. “Take the lotus leaf, for example,” said Ilin Sadeghi, an engineering graduate student in Asatekin’s lab and first author of the study. “The leaf surface is hydrophobic, which means it keeps water off so effectively that the leaf never gets wet—water just beads up on the surface. But it is also very oleophilic—if we place an organic liquid like oil on the surface, it spreads rapidly across the leaf. By modeling on nature, we can engineer surface chemistry and morphology to make water-repelling, super-oleophilic filter materials.”
The lotus leaf achieves its dual behavior with a combination of a waxy surface chemistry and a nanostructured texture on the surface. The textured surface traps air in tiny pockets, making it difficult for water to make contact with the leaf because of water’s high surface tension, forming droplets. Creating an oil-filtering membrane could utilize a similar combination of surface chemistry and texture to separate oil from water.

Lotus leaves provided inspiration for the novel filter material: they are hydrophobic, keeping water off so the leaf never gets wet, and also oleophilic, so organic liquids like oil spread rapidly across the leaf. (Representative image found on Pixabay)
Joined by professor Peggy Cebe and graduate student Nelaka Govinna in the Tufts physics and astronomy department, the research team created a material that combined water-repelling chemistry and texture using a technique called electrospinning.
Govinna, who fabricated the filtration membranes, explains electrospinning as a technique that creates an electrically charged liquid stream of polymer emerging from a very narrow needle. As it flows from the needle, the polymer dries as a fine thread and deposits randomly on the target surface, creating a non-woven, porous web of fibers.
The polymer they used was a chemical chain surrounded by fluorine atoms, which give it water-repelling properties, while the random weave traps air like the lotus leaf to help minimize the penetration of water. By contrast, oily and organic substances flow over the fluoropolymer and through the membrane.
“We created this membrane material by blending a common polymer matrix used in filters—polyvinylidene fluoride or PVDF—with a functional polymer; we call it PFDMA,” said Cebe. “We can change the behavior of the filter membrane by changing the functional polymer.”
In this case, the type of functional polymer provided the membranes with some ideal properties: oil and organic chemicals run rapidly through the membrane, up to seventeen times faster than the PVDF membrane without additive, while water is held back.
The oil-removing PVDF-PFDMA membranes, which allow oil and organic solvents to pass through, don’t foul up like water-removing membranes tend to do, and could therefore be applied to long-term, industrial-scale applications. Using different additive polymers could tune the filter’s properties for different applications, from oil spill cleanups to water purification.
Source: Tufts Now
Media
Taxonomy
- Industrial Wastewater Treatment
- Industrial Water Treatment
- Waste Water Treatments
- Oil Water Separation
- Wastewater Treatment
- Industrial Water Treatment
- Bioremediation
- Remediation
- Oil Refining
- Oil Field Chemicals
- Oil Spill Treatments
- Heavy Oil
- Remediation