Which is the best method for correcting water the pH level ?
Published on by Inamul Choudhury, ALPINE FRESH WATER SYSTEM LTD - Senior Consulting Engineer in Technology
Dear All,
We produce drinking bottle water in 5US gallon poly carbonate bottles and distribute to consumers.
Our reverse osmosis water has pH level of 5.4 -5.5, would it be right to add the caustic solution to enhance pH of water to 6.9-7.4 ? Can we use any other chemical/material without having any health problems? Please suggest some methods for correcting our water pH level.
Media
Taxonomy
- Heath & Safety
- RO Systems
- Quality
- Water Treatment Solutions
23 Answers
-
I suggest that you could try percolating your water through bed of calcite -clean sand mixture. The proportion of calcite and sand can be arrived at by checking the pH of the percolated water. To begin with 70 %sand+30 % calcite mix can be tried.
-
The Water Network research team has consolidated an answer to the above question.
Further input from members is welcome and appreciated.
What is pH?
pH is a numeric scale that indicates the acidity of an aqueous solution.
It is the negative logarithm to base 10 of the molar concentration of hydrogen ions, measured in units of mol/l.
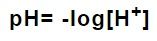
The pH scale has a range from 0 to 14, with the 7 indicating a neutral point.
- Solutions with a pH below 7 are acids.
- Solutions with a pH above 7 are bases.
- Distilled, pure water is neutral, neither acid nor a base, and has a pH of 7.
Pure water is neutral, but when it is mixed with chemicals it can change its acidity. Additionally, mixing acids and bases can neutralize their effects.
Causes of pH variations in water:
- Soil composition through which the water moves, in its bed and as groundwater. Certain rocks can neutralize the acid while others have no effect.
Limestone can buffer – neutralize the acidification of freshwater. - Number of plants and organic matter in the water. Carbon dioxide is released when they decompose, and if forms the carbonic acid as it combines with water. It is a weak acid but in greater amounts in will lower the water pH.
- Chemicals in the water released by industries or individuals. Industrial effluents that are released in the environment, therefore, are required to have a certain pH value.
- Acid precipitation. Acid rain occurs when nitrogen oxides (NOx) and sulfur dioxide (SO2) in the air are combined with water vapor. They are products of car fumes and emissions from coal-fired power plants.
- Coal mine drainage. Sulfuric acid is formed when iron sulfide, which is found around coal mines, is combined with water.
pH standards for drinking water:
Environmental Protection Agency (EPA) maintains strict standards for appropriate pH levels in drinking water. Consuming excessively acidic or alkaline water is harmful, warns the EPA. Drinking water must have a pH value of 6.5-8.5 to fall within the EPA standards , and they further note that even within the acceptable pH range, slightly high- or low-pH water can be unappealing for several reasons. High-pH water has a slippery feel, tastes a bit like baking soda, and may leave deposits on fixtures, according to the EPA website. Low-pH water, on the other hand, may have a bitter or metallic taste, and may contribute to fixture corrosion.
Aquatic life is greatly affected by the water pH. The natural range of most freshwater lakes, streams, and ponds is between 6 and 8.
Most organisms are adapted to a specific water pH and if it is even slightly changed they will die.
If the pH falls below 6, especially 5, it may cause damaging ecological effects.
- As the pH comes close to 5, planktons and mosses – non-desirable species start to invade and some fish populations disappear.
- If the pH is below 5, fish disappear and the bottom gets covered with undecayed matter, with moss is nearshore areas.
- Below 4.5 there is no fish.
The effect of pH on humans:
People have a higher tolerance for pH levels (drinkable levels range from 4-11 with minimal gastrointestinal irritation), there are still concerns.
pH higher than 11 and lower than 4 causes skin and eye irritation.
pH bellow 2.5 causes irreversible skin damage and organ linings.
Lower pH increases the risk of mobilized toxic metals absorption.
Above pH of 8 water cannot be disinfected effectively so other risks may occur.
* Outside the pH range of 6.5-9.5 pipes can corrode and be damaged and thus heavy metals toxicity is increased.
pH adjustment systems:
There are two primary types of system design for pH adjustments – continuous and batch.
Continuous flow
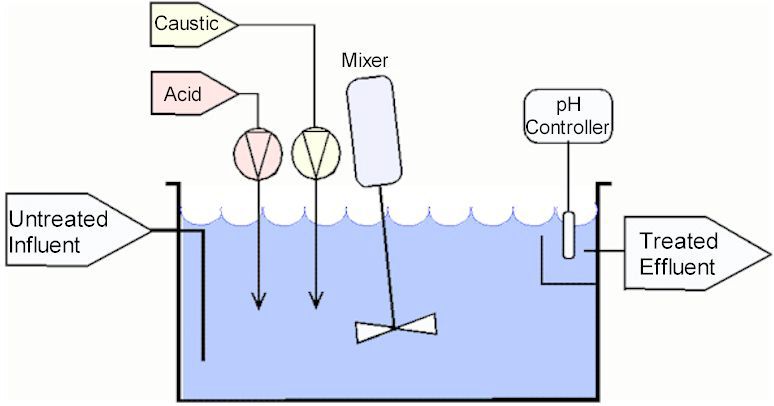 Diagram 1: Continuous flow system.
Diagram 1: Continuous flow system.
Source: phadjustment.comThe tank is constantly full – the amount of influent entering it equal to the treated effluent exiting the tank.
The advantage of this system is that can handle relatively high flows. However, it is not certain that the effluent will always be in range.
Batch
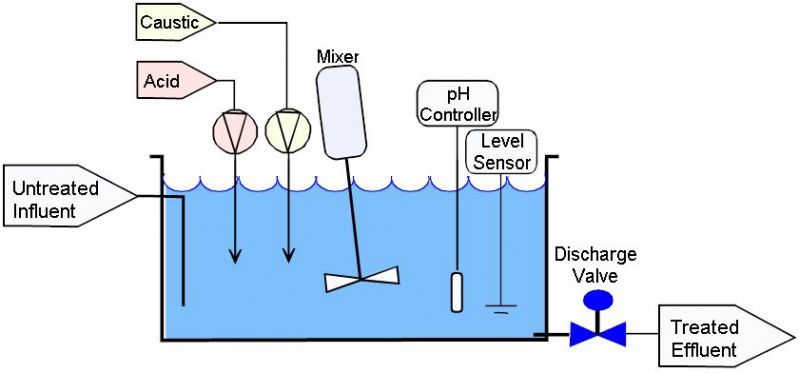 Diagram 2: Batch adjustment system
Diagram 2: Batch adjustment system
Source: phadjustment.comThe batch has a fixed water volume, which is discharged only after fulfilling the criteria.
The influent enters the tank anywhere convenient and exits due to gravity near the bottom, where the port is located.
The batch volume is treated in one cycle.
** The systems shown here are simplified.
pH adjusting methods:
Raising the pH
Lowering the pH
Neutralizing filters
Acid injections
MgO beads
CO2
Soda ash/sodium hydroxide injections
Neutralizing filters
Neutralizing filters are used if drinking water is acidic.
The pH is increased by the addition of the neutralizing material.
It is important to highlight that the water hardness may increase.
(Water hardness is the amount of dissolved calcium and magnesium in the water - dissolved bicarbonate minerals - calcium bicarbonate and magnesium bicarbonate.)Neutralizing filters are point-of-entry devices.
Water with pH greater than 6 is treated with calcium carbonate (limestone) and water with the pH below 6 is treated with the synthetic magnesium oxide.
Untreated water passes through a filter filled with either calcium carbonate or a synthetic magnesium oxide medium and the material dissolves in the water therefore raising the pH level.
The flow rate should not the greater than 2 l/s·m2. The bed should be deep enough to provide sufficient contact time.
The material in the neutralizing filter need refilling and regular backwaching.
If cartridge filters, that retain solids from passing through, are installed before the neutralizing filters, the neutralizing filters will last longer.
After the neutralizing filter a water softener can be added to regulate the water hardness.
The neutralizing filter may result in pressure loss, since the water passes through the finely ground neutralizing material.
The corrosion of the pressure tank and the well pump may occur since the neutralizing filters are installed after the pressure tank.
In case of a high flow rate, liquid injection systems are a better solution.
Magnesium oxide beads
Prill MgO beads are used when the water pH needs to be rasied.
They should be used after reverse osmosis.
Osmosis is a spontaneous movement of the molecules in the solvent through a semi-permeable membrane. The molecules tend to “go” to the in that direction that will equalize the concentrations of the two sides. Reverse osmosis is a process in which the particles move in the opposite direction than in natural osmosis. The contaminated fluid passes through the membrane and the suspended particles are separated from the liquid. For this process, pressure is needed – the hydrostatic pressure needs to be greater than the osmotic pressure.
Prilly Pure Water Beads raise and balance pH levels of the water to 8,7 without any chemicals.
The beads are made from magnesium oxide which is produced from naturally occurring salts of magnesium found in rich brine deposits located approximately 2,500 feet below ground. The resulting magnesium oxide is ‘prilled’ into small, hard pellets by a high temperature firing process which turns them into small ceramic-like pellets.
In addition to adjusting the pH, the beads lower the surface tension of water, remove toxins and pull out heavy metals from water.
Prilly Pure Water Beads last forever and never need to be replaced.
Injection systems:
I Soda ash/sodium hydroxide injection
Soda ash/sodium hydroxide injections are used if the water is acidic.
When injected into a water system, soda ash (sodium carbonate) and sodium hydroxide raise the pH of water.
Injection systems are a point-of-entry system.
Soda ash or sodium hydroxide solution are injected in the water by a corrosion-resistant chemical feed pump.
The injections are installed before the pressure tank so that the tank ant plumbing systems are protected from corrosion.
Dual treatment is used if the water needs to be disinfected, in addition to being neutralized. A chlorine solution is added with the neutralizing chemical.
With the injection systems water with low pH can be effectively treated – as low as 4.
The chemical storage tanks need to be refilled occasionally.
II Acid injection
Acid injection is used for water with a high pH.
Water with a higher pH can have a soda-like taste that is eliminated with this treatment and the chlorination is improved.
Acid injection is a point-of-entry system.
Acid injection reduces pipe corrosion, since water with the pH above 9 corrodes brass, copper, zinc, aluminum and iron.
A solution of acetic acid is injected into water. Usually white vinegar is used, as it is the cheapest, but citric acid and alum are also an option, as well as more hazardous weak solutions of hydrochloric acid or sulfuric acid if the pH is above 11.
The chemicals need to be refilled occasionally, while wearing the protective goggles, gloves and clothing.
Carbon dioxide
Carbon dioxide is used to reduce pH in alkaline water.
Carbon dioxide, CO2, is a colorless and odorless gas. It is a chemical compound composed of a carbon atom covalently double bonded to two oxygen atoms.
It is used as a pretreatment and sulfuric acid is added in the second step. The main purpose of this secondary acidification is to reduce the bicarbonate content and avoid calcium carbonate precipitation.
Carbon dioxide does not corrode the pipes and the equipment.
It was gives better control of pH than sulfuric acid. It shows self-buffering when reaching neutral pH levels. The self-buffering enables precise end-point control eliminating the danger of lowering the pH too much.
It can be utilized via a completely automated system.
Documents on TWN about pH and drinking water standards:
- WHO guidelines for drinking-water quality
- WHO pH in drinking-water
- EPA drinking water standards and health advisories table
- pH Control in WTP by the Addition of CO2
- Drinking Water Treatment - pH Adjustment
- pH Requirements of Freshwater Aquatic Life
- What is pH and How is it Measured?
- pH Theory and Practice
- The Theory of pH Measurement?
-
PrillyPureWater.net (MgO) magnesium oxide beads never dissolve, never wear out and Never needs to be replaced. 8.7 pH is achieved without the use of Any chemical additives, equipment, filters or power sources. Never dissolve s, never wears out and Never needs replacement. Water Freedom Forever! $40 one time investment.
-
Definitely MgO, after RO !!! We use to make it cold on absolute 0, than you can even change some parts of molecular structure !!!!
-
Magnezijum oksid definitivno !!! Naravno, nakon RO!!!
-
There are an estimated 10 million RV owners that you may have some contact with at some level. I have been thinking of a way to get to those RV owners recently. I purify drinking water permanently using magnesium oxide (MgO) in bags for home drinking water containers. The magnesium oxide (MgO) used in the Prilly Pure Water bags actual places a positive charge in the water. This electromagnetic charge shatters the magnetic bond that holds together any Totally Dissolved Solids (TDS), and heavy metals, chlorine, fluoride, lead, copper, black mold, bacteria, E-coli, and also the Emerging Contaminants such as pesticides and pharmaceuticals. These contaminants and pollutants are then gassed out of the container. The pH of the water is raised to 8.7, high alkaline, high oxygen and the water is structured "thin" so as to more readily penetrate the cell membrane and to deliver oxygen, nutrients and pure water and flush out toxins. This is living water. As to hydration, due to it being "thinner" water, 3 glasses of Prilly Pure Water is equal to 1 gallon of tap water. The MgO has been processed at 700 degrees Fahrenheit and made to be more ceramic like a marble in water that never dissolves, never wears out and never needs to be replaced. No expensive equipment, No costly replacement filters, No electricity, and No more bottled water. $40 1-time investment. Regards, Richard Fishman PrillyPureWater.net 808-283-9782
-
Thank you all for offering valued recommendations and comments. I would execute the best one suited to our installation. Still i expect more opinions on the topic. Thanks.
-
Correcting water pH level
Add potassium carbonate (K2CO3) to water. This will increase pH of water as calcium can increase hardness if calcium carbonate is added. Sodium carbonate could be challenging for sodium conscious people. If solutions of potassium carbonate mixed with sodium hypochlorite (K2CO3 + NaOCl) are added to water, this will increase pH and will disinfect water as well because the pH of these chemicals are from pH 10 – 11. If the pH rises too high it can be lowered with acetic acid (white vinegar) to adjust it. Dilute solutions of hydrochloric acid and sulphuric acid can also be used to lower the pH. However, the safe method is to adjust pH with acetic acid. The safe pH for drinking water is from pH 6.0 to 9.0.
-
pH Correction for product water
Dear:
What I would do is just making product water pass through lime beds to accommodate pH to the desired one. Additonally, it will add some minerals to product water that will make it potable for human needs.I hope this will help you in achieving your goal. After that, you will be able to add some quantity of acetic acid
Yours
-
@Inamul, You have some great suggestions here. Compounds of Ca and Mg that will stabilize the pH under various temperature conditions. Avoid adding caustic soda as you would likely have a water with unstable pH conditions, plus there are other issues already mentioned. Best to you!
-
Well noted.
-
Well noted.
-
Post RO Re mineralization with Magnadol, Dolomite or Calcite is ideal for PH control in drinking water
-
Have you considered simply warming the water (if that is an option) after treatment? Warmer water will drive off the dissolved C02 and in some cases the pH change can be dramatic.
-
pH of Water
Dear
If u use NaOH( caustic Soda) to increase the pH level of RO permeate then it can be harmful. It may combine with the fats present in food because it is a strong alkali resulting in soap formation. I suggest you immediately stop this practice
-
I also second reminerilization. Most companies employ this method to RO water before its bottled.
-
Dear all We are getting a ground water ie bore well water with pH:7.0, EC:5.1,HCo3:0.3,Cl:29.2 Ca+Mg: 3.5, Na+K:15.6, SAR:3.71,TDS:3264,Quality:C4S1.These are the water testing reports from the A.P State Govt Lab.We are facing a problem with this water when we irrigate the vegetable crops or feed to the cattle,the plants are not flowering,and the cows are not conceiving,is it due to High E.C or high salinity..can any one help us to solve this problem..can you call us in case if you suggest a remedy kindly contact me by Ph: 09642018373,we are happy send the sample water for further analysis/suggest the right testing lab.We highly appreciate your help. Thank you.
-
Mixed bed is the best option for ph correction. No other way out.
-
Hi Inamul, Remineralization with CaCl2 + MgCl2 and KHCO3 is considered best for drinking. Remineralization will also adjust the pH. Caustic soda is not recommended. Other method is to blend raw water with proper treatment ( as necessary based on impurities present) but let me caution you that this is not the best method.
-
I would echo Patricks comment, favouring use of a mineral such as calcium carbonate to increase the pH stability of the water (buffering capacity). In the UK, utilities sometimes use a combination of hydrated lime (calcium hydroxide) and carbon dioxide to achieve this.
-
RO does not remove dissolved CO2 and that makes the water acidic. I would not recommend to add any chemical to raise pH. Just try to increase the TDS of your RO water a little to a point when you get 6.5 pH minimum, that is the EPA minimum pH for drinking water. Increased TDS will add total alkalinity to treated water and correct the pH.
-
Dear Inamul, First, what is your source of raw water ? Is it seawter or Brackish water ? Furthermore, in most cases water coming from RO should not be used as drinking water directly as concentration of some minerals are too low, especially magnesium and calcium (there are recommendations of the WHO). In most cases, you should proceed for a remineralisation, which will also correct your pH. There are different solutions, most often using calcium based rock (dolomite or calcite). I would not recommend using caustic soda. Best regards.
-
Hi Inamul, We have recently released report on US pH meter Market Forecast & Opportunities, 2020, I hope you would certainly find answer of your query in that. Kindly write me on kalpana.verma@techsciresearch.com to know more about the report. Regards, Kalpana