Desalination Methods
Published on by Marina A, Previously Key Account and Content Manager at AquaSPE AG in Academic
Desalination is the separation of dissolved salts and other minerals from water.
This water can be seawater, brackish water, rivers and streams, wastewater, industrial feed and process water.
Seawater desalination is a promising solution to the water shortage problem since seawater covers more than 70% and represents 97% of the total amount of water on earth. Moreover, it is one of the rare water sources independent from rainfall.
Methods:
· Multi-Stage Flash Distillation
· Multiple Effect Distillation
· Vapor Compression Distillation
· Reverse Osmosis
· Freezing
· Solar Evaporation
· Electrodialysis/Electrodialysis Reversal
Multi-Stage Flash Distillation
Multi-Stage Flash Distillation (MSF) is the most commonly used desalination method, producing 60% of all desalinated water.
In MSF vaporization occurs at low temperatures in a vacuum and the vapor condenses, providing fresh water. Since the pressure in a vacuum is lower, lower temperatures are needed for boiling.
MSF flashes a portion of water into stream in multiple stages of countercurrent heat exchangers.
MSF plants are based on the principles of heat exchangers and condensate collectors.
There is a hot and cold end with intermediate temperatures between them.
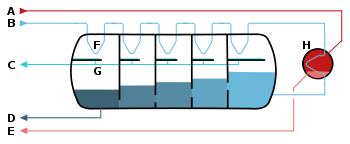
Image source: https://en.wikipedia.org/wiki/Multi-stage_flash_distillation
Image: Schematic of a 'once-through' multi-stage flash desalinator
A - Steam in
B - Seawater in
C - Potable water out
D - Waste out
E - Steam out
F - Heat exchange
G - Condensation collection
H - Brine heater
Cold seawater passes through condensing coil in vacuum flash chambers to get preheated and to condense the steam to fresh water.
Preheated water is then heated with a brine heater to 90-110 ֯C. This heated water goes in the flash chamber at a vacuum. This water has a higher temperature than the boiling point in a vacuum so a flash of water is turned to steam. This steam rises in the chamber where it condenses to pure water when it comes in contact with condensing coils.
When the water enters, steam is formed; and as more vapor forms, the pressure increases which reduces the evaporation and increases condensation.
The brine remains at the bottom still and contains salts and impurities.
This process repeats successively in the multiple chambers – each chamber having lower pressure and temperature than the previous one, lowering the boiling pressure.
MSF plants can have from 4 to 40 stages.
MSF can get 20% of fresh water from seawater and the brine is returned to the sea.
Disadvantages: high temperatures may contribute to and cause scale formation and corrosion.
Multiple Effect Distillation
Multiple Effect Distillation (MED) evaporates and distills seawater in multiple stages while reusing the energy from vapor condensation.
Total energy consumption for water heating is reduced due to energy reuse.
Water is evaporated at lower temperatures, < 70 ֯C.
MED is a sequence of closed spaces with successively lower pressures and temperatures.
Each sequence has a heat source for the previous one and a heat sink which leads to the next sequence.
The horizontal tubes in each stage are sprayed and cooled with the seawater make-up flow.
The heating system is inside the tubes and the steam condensates into pure water inside the tubes. Simultaneously, the seawater gets heated on the outside of the tubes gets heated and evaporates.
Only the first effect is heated from the external source of steam.
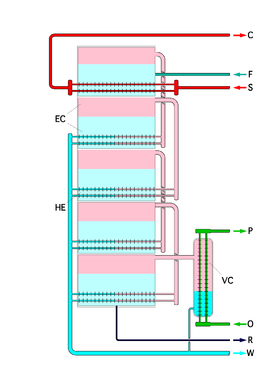
Image source: https://en.wikipedia.org/wiki/Multiple-effect_distillation
Image: Schematic of a multiple effect desalination plant
Feed water (F) enters the first stage and is then heated by an external steam (S). The water then evaporates (pink area) and the steam is directed by pipes to the next stage where the water (blue area) is heated by that steam and the process repeats till the last stage.
F - feed water in
S - heating steam in
C - heating steam out
W - Fresh water (condensate) out
R - brine out
O - coolant in
P - coolant out
VC is the last-stage cooler
Multiple Effect Distillation is a self-explanatory name since this process gets repeated in a series of cells (effects).
Amount of distilled water in every stage is directly proportional to transported energy.
To increase the distilled water production, the surface area per stage can be increased.
Advantages:
· reduced scaling risk
· small and medium size plants
· low thermal energy consumption and reduced operating costs
Vapor Compression Distillation
Vapor Compression Distillation (VCD) is a one-step process in which water is heated and evaporated and then compressed and condensed to pure water.
Water is compressed by a blower, compressor or jet ejector. The compression provides a heat transfer.
Thee compressor is the main power consumer.
By compressing, the pressure is increased which also increases the boiling point temperature, since the volume and temperature are directly proportional according to the Ideal Gas Law.
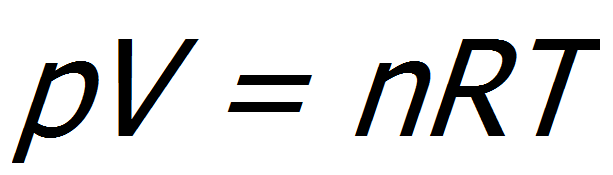
Formula: Ideal Gas Law
p – gas pressure
V – gas volume
n – amount of substance of gas (in moles)
R – ideal gas constant
T – gas temperature
The vapor can be used to further heat the liquid the vapor evaporated from.
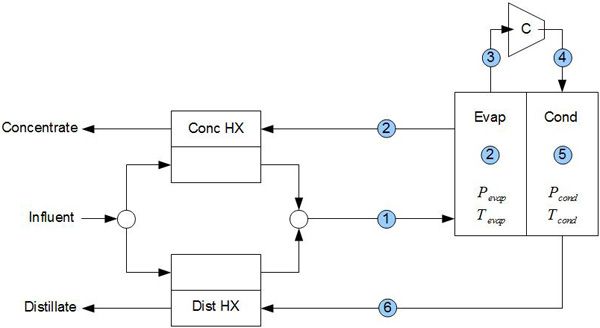
Image source: http://www.aquaback.com/technology/how-it-works
Image: Vapor Compression Desalination
1. Incoming water (influent) is heated by the distillate (pure water) and concentrate (grey water).
2. Influent is evaporated to steam. Contaminants are left on the evaporator surface and are collected as concentrate which exits the system while heating the influent
3. The steam is compressed
4. Hot steam is sent to the condenser chamber
5. Steam is condensed producing pure water
6. The distillate is cooled down due to heat exchange with the influent
If compression is done by a mechanically driven compressor, the process is usually called mechanical vapor recompression (MVR).
Advantages:
· lower operating costs than MSF and MED
· smaller equipment than MSF and MED
Disadvantages:
· compressor and heat exchange maintenance
· high energy consumption
· high capital costs
Reverse Osmosis
Reverse osmosis (RO) is a desalination process which uses semipermeable membranes and applied pressure to purify the water from salts.
Two fluids with different concentrations (e.g. water with different salt concentrations) will tend to achieve an equilibrium and even the concentrations.
Osmotic pressure is the minimum pressure which will stop the inflow of one fluid to another through a semipermeable membrane.
Reverse osmosis applies pressure to overcome osmotic pressure, waking the fluid go in the opposite direction.
RO uses less energy than thermal desalination processes.
In RO desalination, seawater passes through a membrane due to applied pressure and gets rid of the salts.
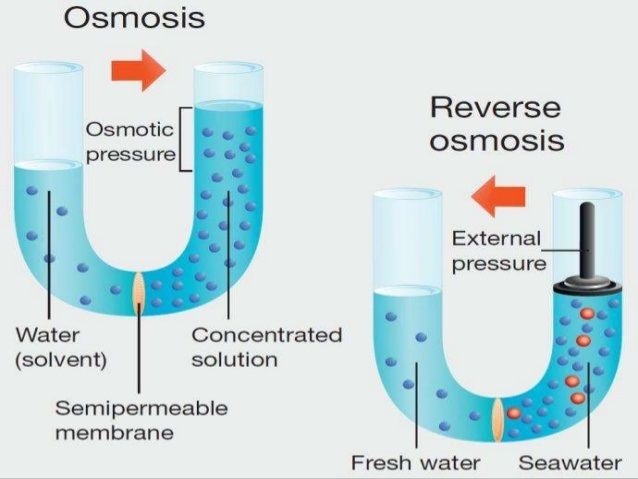
Image source: https://www.slideshare.net/wwwtwastewater/reverse-osmosis-desalination-water-treatment
Image: Osmosis and Reverse Osmosis
Freezing
When seawater is frozen, water gets separated from the salt so that the water can properly crystallize.
Seawater is partially frozen and the ice is removed and then melted into pure water.
Solar Evaporation
In solar evaporation the natural water cycle principle is applied. Water is heated and evaporated by the sun. Condensated water vapor is separated and cooled down to provide pure water.
Electrodialysis/Electrodialysis Reversal
Electrodialysis (ED) utilizes salt ion movement to desalinate water by applying an electric filed and ddirecting the ions through a membrane.
ED transports salt ions from one solution to another through a ion-exchange membrane due to the electric potential difference.
If direct current is applied to the water, the negatively charged chloride ions will move to the positively charged anode, and positively charged sodium ions will move to the negatively charged cathode.
In electrodialysis, the ions move through membranes and create two separate streams – the desalinated and concentrated stream.
Usually mltiple electrodialysis cells are arranged with alternating anion and cation exchange membranes.
Electrodialisis is a cheap and fast desalination method but it does not remove contaminants and bacteria.
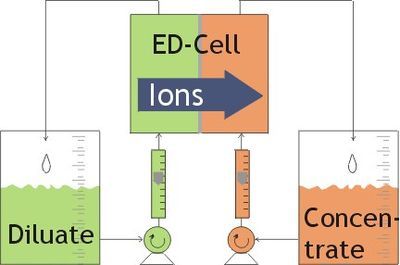
Image source: https://en.wikipedia.org/wiki/Electrodialysis
Image: Electrodialysis scheme
Sources:
- https://en.wikipedia.org/wiki/Desalination
- http://www.brighthubengineering.com/power-plants/29623-how-desalination-by-multi-stage-flash-distillation-works/
- https://en.wikipedia.org/wiki/Multi-stage_flash_distillation
- http://www.entropie.com/en/services/desalination/MED/
- https://en.wikipedia.org/wiki/Vapor-compression_evaporation
- https://people.uwec.edu/piercech/desalination/VCD.htm
- http://www.aquaback.com/technology/how-it-works
- https://en.wikipedia.org/wiki/Electrodialysis
Media
Taxonomy
- Desalination
- Thermal Desalination
- Solar Desalination
- Sea Water Desalinisation
- Sustainable Desalination
- Desalination