Finding Value in Textile Wastewater
Published on by Trudi Schifter, CEO and Founder AquaSPE in Technology
Finding Value in Textile Wastewater

Nitrogen and sulfur present in dye-contaminated wastewater are incorporated into an energy material, simultaneously addressing two challenges.
IMAGE CREDIT: NAOMI KOELEMANS ON UNSPLASH
According to the UN, more than 80 per cent of wastewater caused by human activities is discharged without any pollution removal. The textile industry is no exception—synthetic dyes are used on a massive scale in this heavily polluting industry, and inefficiencies in the dyeing process mean that dyes end up not only in our clothing as intended but in our waterways, posing a major threat to both the aquatic environment and human health.
Currently, the most widely used methods of removing dye from wastewater are flocculation precipitation and adsorption. However, both methods produce large quantities of sludge, which ends up being disposed of in landfills or by incineration and causes secondary pollution.
Given these issues, the management of wastewater containing dyes, which can persist for long periods in the environment and contaminate drinking water, is a global challenge that requires sustainable solutions.
A team of researchers have addressed this issue by not only treating the dye wastewater but also finding an innovative use for it. Industrial dye wastewater is rich in nitrogen and sulfur from the azo and sulfite functional groups present in the dyes, and these elements can act as heteroatom dopants in carbon materials for energy storage devices.
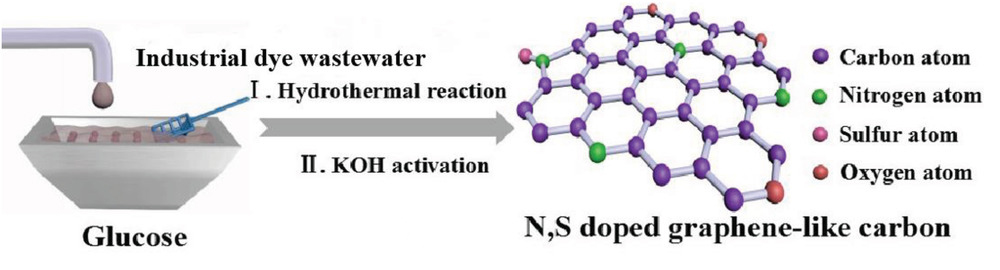
Schematic illustration of N,S‐doped graphene‐like carbon extracted from wastewater.
To extract this added value, the researchers subjected the dye-containing wastewater to a hydrothermal reaction with glucose and then chemical activation with potassium hydroxide. This one-pot synthesis yielded a porous carbon material with moderate heteroatom doping, high specific surface area, and a high degree of graphitization—all beneficial criteria for an electrode in a supercapacitor cell.
Testing the electrochemical performance of a cell based on this electrode material confirmed that it is indeed a promising candidate for energy storage applications. In support of a circular economy, the researchers hope to extend their concept to other areas, including general wastewater treatment, air pollution control, and carbon capture, storage, and utilization.
Nitrogen and Sulfur Co‐Doped Graphene‐Like Carbon from Industrial Dye Wastewater for Use as a High‐Performance Supercapacitor Electrode
Abstract from WILEY online Library
Nitrogen and sulfur co‐doped graphene‐like carbon (N,S‐GLC) is successfully prepared in a one‐step hydrothermal reaction of glucose with industrial dye wastewater followed by chemical activation. The nitrogen and sulfur are sourced entirely from the industrial wastewater. The process not only provides an alternative way of treating industry wastewater, but also offers a green route for recovering energy from the waste in the form of chemicals. The resultant N,S‐GLC shows a good degree of graphitization, a high specific surface area (1734 m2 g−1), and moderate heteroatom doping (N: 2.1 at%, S: 0.7 at%). The N,S‐GLC electrode displays high specific capacitance of 275 F g−1 at a current density of 0.5 A g−1 with a retention of 65.4% at 20 A g−1 in 6 m KOH. Moreover, the assembled symmetrical supercapacitor cell shows a capacitance of 38 F g−1 at a current density of 0.5 A g−1, which is equivalent to an energy density of 6.4 Wh kg−1 at a power density of 275.0 W kg−1. This approach provides an alternative and sustainable way of fabricating heteroatom‐doped graphene‐like carbon materials for use in high‐performance supercapacitors.
1 Introduction
Due to increased energy demands and intensified environmental concerns worldwide, there is an urgent need to develop eco‐friendly, low cost, and high‐performance energy storage devices.1-3 The supercapacitor is considered to be a promising energy storage device because of its fast charge and discharge, long cycle stability, and high power‐density compared with other storage devices.4, 5 Supercapacitors can be divided into pseudocapacitors and electrical double‐layer capacitors (EDLCs) based on their different charge storage mechanisms.6 EDLCs depend on the reversible adsorption and desorption of electrode ions at the interface between the electrode and the electrolyte. Until now, tremendous effort has been devoted to improving the capacity of supercapacitors by developing new kinds of electrode materials, including carbon‐based materials.7,8
Carbon compounds, such as graphene,9, 10 carbon nanotubes,11 and porous carbons,12-14 have received extensive attention as electrode materials in EDLCs. Given the need for the development of cost‐effective, well‐performing electrode materials,15 carbon materials that have a high specific surface area and hierarchical porous structures are an efficient way of obtaining satisfactory electrochemical performance when used as the electrodes in EDLCs.16, 17 Additionally, heteroatom doping with nitrogen (N) and sulfur (S) can enhance the capacitance by promoting the wettability and pseudocapacitive properties of the specialized carbon materials.18, 19 At present, the preparation of N and S co‐doped porous carbon is achieved by using N and S containing compounds. Recently, Li et al. reported fabricating a porous carbon using a potassium hydroxide (KOH)‐activated willow‐catkin process followed by N‐ and S‐doping using thiourea. The doped carbon had a specific capacitance of 249 F g−1 at 0.5 A g−1 in a 6 m KOH electrolyte.20 Kong et al. reported on N and S co‐doped graphene using ammonium thiocyanate (NH4SCN), which delivered a capacitance of 209 F g−1 at 10 A g−1 in a 6 m KOH electrolyte.21 Despite the beneficial increase in capacitance this type of doping produces, the current method for the preparation process is generally complex, time‐consuming, and results in low yields, which has largely limited its use to industrial applications. In addition, synthetic chemicals have been the main source of N and S dopants in previous methods, which increases the resource input required for manufacturing doped carbon materials. Therefore, the production of high‐performance, functional, porous carbon using green and efficient methods using renewable and sustainable feedstocks is a highly desirable future direction for the manufacturing of supercapacitors.22-24 Our approach has several advantages: 1) this method provides an alternative way of treating industry wastewater when producing useful doped carbon materials; 2) it offers a green route of recovering energy from the waste; 3) no additional N and S containing chemicals are required to produce doped porous carbon in our approach. With N and S dopants, new electrochemically active sites are generated. The Faraday reaction of the surface N, S functional group can increase the specific capacitance of the porous carbon material.
In recent years, an increasing demand for synthetic dyes has resulted in large amounts of wastewater generated by the textile and dyeing industries. This wastewater is composed mainly of aromatic heterocyclic compounds, which are difficult to degrade and harmful to human health,25, 26 meaning it is necessary to pretreat the wastewater before discharging to the environment. Adsorption is the most widely used method for treating this type of wastewater.27 In previous reports, research has focused on using various kinds of activated carbon as the adsorbents,28, 29 while little effort has been devoted to recovering energy from wastewater. The organic compounds that are rich in N and S present in the wastewater could support a new route of treatment that yields energy while preparing heteroatom‐doped graphene‐like carbon with the heteroatoms from the dye wastewater.
Herein, we present a new approach to generate graphene‐like porous carbon that is co‐doped with N and S via a hydrothermal reaction and subsequent chemical activation of the glucose in the dye wastewater, and its potential application to energy storage technologies. The surface of the glucose molecule contains a large amount of –OH, making it compatible with many organic substances and be able to grab most of the organic pollutants during the synthesis and convert them to the useful dopants in the porous carbon. Compared with previous methods for making heteroatom‐doped porous carbon, our approach and strategy has the following features: 1) simultaneous treatment and recovery of useful components (such as N and S) from dye wastewater; 2) the elimination of the requirement of additional chemicals to act as N and S sources in order to prepare the heteroatom‐doped carbon; 3) providing a new approach for dye wastewater treatment with a value‐added function; and 4) the resultant carbon material possesses 3D hierarchical structures with a high specific surface area, moderate N/S contents, and a high degree of graphitization, all of which provide a better material for use in energy storage systems.
2 Results and Discussion
The integration of industrial wastewater into the production of N,S‐doped graphene‐like carbon is schematically illustrated in Figure 1a. The dye wastewater used in this study contained mainly azo dye compounds, sulfite (SO32−) functional groups, and organic materials that are rich in N and S.30, 31 The dye wastewater was mixed with glucose prior to the hydrothermal reaction. After the heat treatment, the organic and N/S atoms deposited on the glucose‐based carbon. The dehydration of glucose to oligosaccharides and aromatic compounds occurred mainly at temperatures below 140 °C. Above 160 °C, the oligosaccharide molecules undergo intermolecular dehydration to form a crystal nucleus. These crystal nuclei continue to undergo polymerization and eventually form carbon spheres with active functional groups, such as hydroxyl and carbonyl groups, on the surface. These functional groups not only increase the hydrophilicity of carbon materials, but also adsorb some of the ions in the industrial wastewater to form composite carbon materials. KOH activation was then used to generate more porosity and promote the degree of graphitization.32 The reaction between KOH and carbonaceous material can be primarily expressed as
6KOH+2C→2K+3H2+2K2CO36KOH+2C→2K+3H2+2K2CO3

Figure 1
Open in figure viewerPowerPoint
a) Schematic illustration of N,S‐doped graphene‐like carbon. b) XRD patterns of a‐carbon and N,S‐GLC. c) Raman spectra of a‐carbon and N,S‐GLC. d) Nitrogen adsorption–desorption isotherms of a‐carbon and N,S‐GLC. e) Pore size distribution analyzed by the NLDFT method.
Media
Taxonomy
- Industrial Wastewater Treatment
- Industrial Water Treatment
- Dyes & Pigments
- Textile