Improving the Sensitivity for Ionic Solutes Analysis
Published on by Water Network Research, Official research team of The Water Network in Academic
Researchers from Kumamoto University in Japan have developed a new method to improve the sensitivity of analytical systems for ionic solutes, such as water from rivers and lakes, or even tap water.
Clean water is important for community health, and impurity analysis can is conducted via sensitive equipment such as mass spectrometers. However, these devices can be prohibitively expensive for low-income areas or if only a few samples are to be analyzed. In these cases, an enrichment process is used to improve the sensitivity of more easily accessible analytical systems.
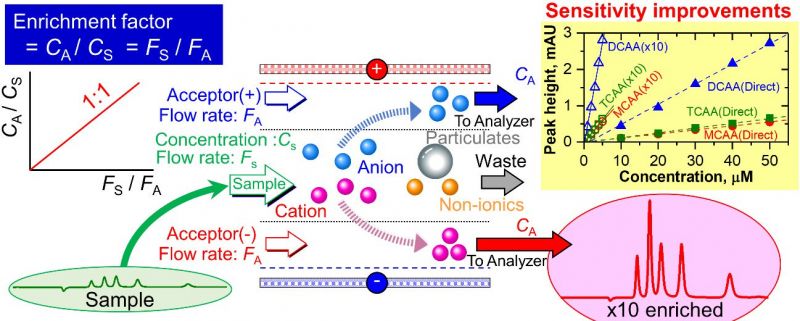
To increase the detection sensitivity of ionic solutes in a water sample, it is enriched via electrodialytic ion transfer before it passes to the analyzer.[Reprinted from Talanta, 180, Shin-Ichi Ohira, Takayuki Yamasaki, Takumi Koda, Yuko Kodama, and Kei Toda, Electrodialytic in-Line Preconcentration for Ionic Solute Analysis, 176-181, (April 2018) with permission from Elsevier. doi: 10.1016/j.talanta.2017.12.054] Credit: Dr. Shin-Ichi Ohira
The limits of detection of trace ionic solutes, such as those left over after purification, should be at least a few micrograms per liter and even less for ultrapure water used in industrial processes. There are several enrichment methods that can be used to increase ionic solute levels in a sample to improve analysis sensitivity, such as heating with/without a vacuum, nitrogen-flow evaporation, co/precipitation for heavy metal ions, liquid-liquid extraction, solid phase extraction, or electrodialysis.
The researchers used the electrodialytic ion transfer enrichment method since quantitative ion transfer can be achieved and the transferred ions become enriched if the sample solution flow rate is higher than the acceptor solution.
They found that if the flow rate for the sample solution (Fs) was higher than that of the acceptor solution (Fa), the enrichment effect became equivalent to the ratio of the two flow rates (when Fa is not 0), and ion enrichment could be performed in just a few seconds.
Upon testing this enrichment method, researchers found that the ion chromatography system detection limits were improved by a factor of ~10 for inorganic cations and ~50 for heavy metal ions, which follows the flow rate ratios of Fs/Fa = 10 and 50 respectively. They achieved similar detection limit results in a flow injection analysis (FIA) system. With the present method, the sensitivity of any analytical system can be improved.
Finally, the byproducts of drinking water chlorination, chloroacetic acid (MCAA), dichloroacetic acid (DCAA), and trichloroacetic acid (TCAA) were assessed in Japanese tap water with a HPLC-UV detector using the researcher's electrodialytic enrichment process. The analysis required a relatively higher voltage than that for strong acids to obtain a flow rate ratio of 10, and enrichment factors for the tap water ions averaged an acceptable 9.8.
"Our in-line, real-time, enrichment of ionic solutes improves the sensitivity of testing systems," said Professor Shin-Ichi Ohira, leader of the research project. "What's more, it can be done in just a few seconds. We envision this pretreatment to be implemented in future, fully automated systems."
Source: Kumamoto University (Via Phys.org)
More information: Shin-Ichi Ohira et al, Electrodialytic in-line preconcentration for ionic solute analysis, Talanta (2017). DOI: 10.1016/j.talanta.2017.12.054

Representative image, source Max Pixel
Media
Taxonomy
- Drinking Water Security
- Treatment
- Chemical Treatment
- Biological Treatment
- Drinking Water Treatment
- Resource Management
- Drinking Water
- water treatment