Using soap to remove micropollutants from water
Published on by Water Network Research, Official research team of The Water Network in Technology
MIT chemical engineers create affordable, sustainable soap-based system to eliminate emerging micropollutants in water. 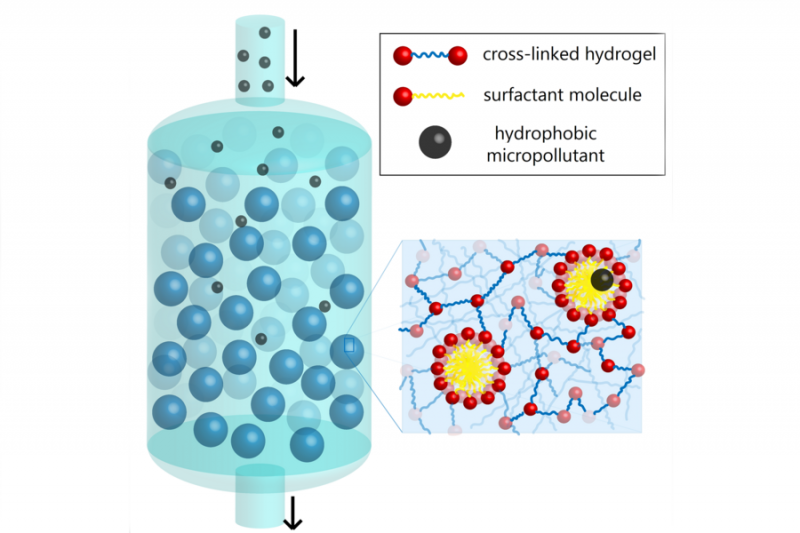
Illustration of water molecules running through hydrogel particles in red and surfactants in yellow
Caption:Schematic diagram of micelle-laden hydrogel particles in a packed bed for the removal of micropollutants. Credits:Image courtesy of the Doyle Lab.
Imagine millions of soapy sponges the size of human cells that can clean water by soaking up contaminants. This simplistic model is used to describe technology that MIT chemical engineers have recently developed to remove micropollutants from water — a concerning, worldwide problem.
Patrick S. Doyle, the Robert T. Haslam Professor of Chemical Engineering, PhD student Devashish Pratap Gokhale, and undergraduate Ian Chen recently published their research on micropollutant removal in the journal ACS Applied Polymer Materials. The work is funded by MIT’s Abdul Latif Jameel Water and Food Systems Lab (J-WAFS).
In spite of their low concentrations (about 0.01–100 micrograms per liter), micropollutants can be hazardous to the ecosystem and to human health. They come from a variety of sources and have been detected in almost all bodies of water, says Gokhale. Pharmaceuticals passing through people and animals, for example, can end up as micropollutants in the water supply. Others, like endocrine disruptor bisphenol A (BPA), can leach from plastics during industrial manufacturing. Pesticides, dyes, petrochemicals, and per-and polyfluoroalkyl substances, more commonly known as PFAS, are also examples of micropollutants, as are some heavy metals like lead and arsenic. These are just some of the kinds of micropollutants, all of which can be toxic to humans and animals over time, potentially causing cancer, organ damage, developmental defects, or other adverse effects.
Micropollutants are numerous but since their collective mass is small, they are difficult to remove from water. Currently, the most common practice for removing micropollutants from water is activated carbon adsorption. In this process, water passes through a carbon filter, removing only 30 percent of micropollutants. Activated carbon requires high temperatures to produce and regenerate, requiring specialized equipment and consuming large amounts of energy. Reverse osmosis can also be used to remove micropollutants from water; however, “it doesn't lead to good elimination of this class of molecules, because of both their concentration and their molecular structure,” explains Doyle.
Inspired by soap
When devising their solution for how to remove micropollutants from water, the MIT researchers were inspired by a common household cleaning supply — soap. Soap cleans everything from our hands and bodies to dirty dishes to clothes, so perhaps the chemistry of soap could also be applied to sanitizing water. Soap has molecules called surfactants which have both hydrophobic (water-hating) and hydrophilic (water-loving) components. When water comes in contact with soap, the hydrophobic parts of the surfactant stick together, assembling into spherical structures called micelles with the hydrophobic portions of the molecules in the interior. The hydrophobic micelle cores trap and help carry away oily substances like dirt.
Doyle’s lab synthesized micelle-laden hydrogel particles to essentially cleanse water. Gokhale explains that they used microfluidics which “involve processing fluids on very small, micron-like scales” to generate uniform polymeric hydrogel particles continuously and reproducibly. These hydrogels, which are porous and absorbent, incorporate a surfactant, a photoinitiator (a molecule that creates reactive species), and a cross-linking agent known as PEGDA. The surfactant assembles into micelles that are chemically bonded to the hydrogel using ultraviolet light. When water flows through this micro-particle system, micropollutants latch onto the micelles and separate from the water. The physical interaction used in the system is strong enough to pull micropollutants from water, but weak enough that the hydrogel particles can be separated from the micropollutants, restabilized, and reused. Lab testing shows that both the speed and extent of pollutant removal increase when the amount of surfactant incorporated into the hydrogels is increased.
“We've shown that in terms of rate of pullout, which is what really matters when you scale this up for industrial use, that with our initial format, we can already outperform the activated carbon,” says Doyle. “We can actually regenerate these particles very easily at room temperature. Nearly 10 regeneration cycles with minimal change in performance,” he adds.
Taxonomy
- Pollutants
- Micropollutants