Water Treatment Membranes and Their Processes by Fluence
Published on by Laura Silvello, Marketing Manager, Italy Operations at Fluence Corporation in Technology
Filtering out contaminants, even at the nanoscale, membranes are a mainstay of water and wastewater treatments.
In water treatment, membranes are barriers that allow water to pass through but stop unwanted substances from passing through with it. Working much like the cell walls in our bodies, technical membranes filter out salts, impurities, viruses, and other particles from water.
A membrane process is any method that relies on a membrane barrier to filter or remove particles from water. The fluid is passed through the membrane because of the pressure difference between one side of the membrane and the other. Contaminants remain on one side. Although many types of filter media are used for water treatment — for instance, clay, silt, and sand — one of the properties that distinguish membranes is their ability to separate smaller substances such as salts and ions from a liquid.
Membranes were first applied to water treatment processes in the 1960s, but in the next decade, they became increasingly used for desalination. Now, the list of membrane processes used in water treatment has lengthened to include:
- Forward osmosis
- Reverse osmosis
- Microfiltration
- Ultrafiltration
- Nanofiltration
Different processes require different types of membrane, broadly speaking, either functioning as a sieve or separating water from impurities on a molecular level. Membranes are made from polymer-based films, ceramics, and other materials. Research is underway on materials block polymers, aluminum oxide, graphene, and other nanomaterials like carbon nanotubes. Membranes have varying degrees of permeability: MF membranes have the largest pore size at 0.1 to 10 microns, followed by UF at 0.1-0.01 microns, NF at 0.01-0.035-microns, and RO membranes, which effectively are nonporous at 0.0001 of a micron.
.jpg)
Two membrane processes — ultrafiltration and reverse osmosis — are used to treat silty river at the Central Puerto facility in Buenos Aires, Argentina.
Membrane Types and Configurations
Membranes usually are classified as isotropic or anisotropic. Isotropic membranes show a uniform composition and physical structure in cross-section, while anisotropic membranes are not uniform in cross-section. They generally are formed from differently structured layers and different materials.
Types of membranes in general use include tubular, hollow-fiber, and flat-sheet. These types are applied in different configurations, such as within a frame, like the flat-sheet membranes used in Fluence’s Smart Packaged NIROBOX™ solution, or spirally wound, such as those used in membrane aerated biofilm reactor (MABR) technologies like Fluence’s Aspiral™ Smart Packaged wastewater treatment plants.
The ideal properties of water treatment membrane configurations are:
- Compactness
- Low tangential flow resistance
- Uniform velocity distribution without dead regions
- High retentate-side turbulence to minimize fouling and help mass transfer
- Easy maintenance and cleaning
- Low unit cost
David C. Sammon explains membranes’ capabilities in his paper Membrane Processes:
"In general, membrane processes offer the possibility of separating water from various types of solute and of separating solutes either on the basis of size or because some are ionized and others are not. In addition to these cases where a high degree of separation is achieved, there are many instances where the composition of the dissolved material is altered. One example is in reverse osmosis, where the permeate has a considerably reduced salt content."
Forward and Reverse Osmosis
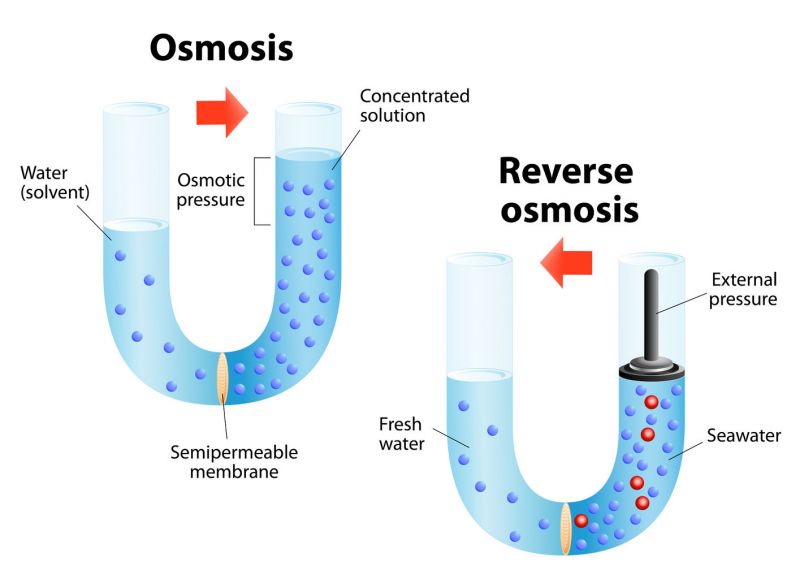
In water purification, membranes are used in osmosis and reverse osmosis, among other processes. (Image source: designua/123RF)
Forward osmosis, or simply osmosis, is a physical process in which a solvent moves across a semipermeable membrane. It is best known as the process cells use to transport water. Water is present on both sides of the membrane, with different levels of dissolved minerals on both sides. The water with the greater concentration of solutes naturally becomes diluted. Jean-Antoine Nollet, a French scientist, and cleric, first observed osmosis in 1748 and coined the term based on the Greek words, endosmose and exosmose.
Reverse osmosis (RO), in contrast, relies on pressure to force water through a membrane, thus separating water from impurities. A 2018 survey of industry professionals on the effectiveness of water reuse technologies placed RO among the top-ranked. While RO is frequently used for desalination, it also is used for wastewater treatment and water reuse, as well as for the removal of trace phosphates, calcium, heavy metals, and other substances.
Microfiltration and Ultrafiltration
In membrane technologies based on blocking particles — including microfiltration and ultrafiltration — pore size is important because it determines the size of the particles and microorganisms that can pass through the barrier. The small-pored membranes used in ultrafiltration block proteins, fatty acids, macromolecules, bacteria, protozoa, viruses, and suspended solids.
Membrane Process Challenges
The effectiveness of membrane treatment often depends on the condition of the membrane. For example, for reverse osmosis technologies to operate efficiently the membrane must be impeccably maintained or it can be fouled with scale or biofilms, a perennial problem. Fouling can reduce effectiveness and increase energy consumption. Much research is devoted to engineering membranes to resist fouling through specialized coatings or other treatments, such as changing the charge of the membrane material.
In the mid-2010s, researchers in Israel developed an important chemical-free process to prevent membrane fouling in RO desalination. The process prevents membrane fouling, lowers chemical costs, and makes desalination more eco-friendly. Pretreatment with a two-stage, granular rapid bioflocculation filter (RBF), a first-stage bioflocculator (BF), and a mixed-media bed filter (MBF), prevents fouling agents from reaching the membrane.
This and other improvements to seawater reverse osmosis (SWRO) have made the process much more cost-efficient, which in turn has led to explosive global growth in SWRO.
Harnessing Biofilms’ Properties
Membranes used in wastewater treatment and reuse, too, are vulnerable to biofilm fouling. Formation of biofilm on filtration membranes and resultant clogging of pores (biofouling) is one of the most stubborn problems faced by such operations.
In some applications, 85ºC sanitization of membranes is used for chemical-free maintenance. In a membrane aerated biofilm reactor (MABR), however, biofilm actually is harnessed to do the heavy lifting in the treatment process. The membranes used in MABR, a biological treatment, are semipermeable at the molecular scale to aid in aeration without bubbles, which allows robust growth of helpful microorganisms.
The vertical, spirally wound aerating membrane is simply flushed from the bottom with bubbles periodically, which is sufficient to prevent a clogging biofilm overgrowth without mechanical mixing.
Combined Treatments
Membrane processes are frequently combined with other processes to provide comprehensive water treatment solutions. For instance, the Central Puerto facility in Buenos Aires, Argentina, needed to treat river water before its use in industrial equipment. Ultrafiltration was used in combination with reverse osmosis to create demineralized water for the plant’s high-pressure boiler. The ultrafiltration process helped membrane fouling issues.
Mine water treatment is another example of combined processes. Typically, wastewater from mining operations is extremely high in total suspended solids and colloids. Ultrafiltration can remove these particles to prepare it for treatment with reverse osmosis. In some cases, the water is passed through reverse osmosis twice to reach the final specifications for full water treatment.
Contact Fluence to learn more about our advanced membrane process-based solutions for your unique source water challenges. Click here to read the original article.
Media
Taxonomy
- Membranes
- Water Treatment Solutions
- Membrane Technology
- Membrane Filtration
- Membrane
1 Comment
-
I need a very good Membrane technology company to possibly partner for Nigeria and African Market
1 Comment reply
-
Dear Bibilore, please fill in the form here https://www.fluencecorp.com/contact/ and a sales representative will get back to you as soon as possible.
-