Yale engineers invent water purification device based on Mangrove trees capillary action
Published on by Water Network Research, Official research team of The Water Network in Academic
Mangrove trees are like no other — they can remove salt from seawater using a complex filtration system, which allows them to grow in places that other plants cannot.
Engineering professor Menachem Elimelech and his team have created a device that mimics the desalination capabilities of mangrove trees. The success of the device in purifying brackish water will pave the way for future studies on water transport in plants. In addition, the device shows promise for other environmental innovations.
“The principles established by the device could be used in a variety of applications, including dewatering of concentrated brines, environmental sensors and smart infrastructure,” Jay Werber GRD ’16 ’18, a project team member who performed preliminary experiments, wrote in an email.
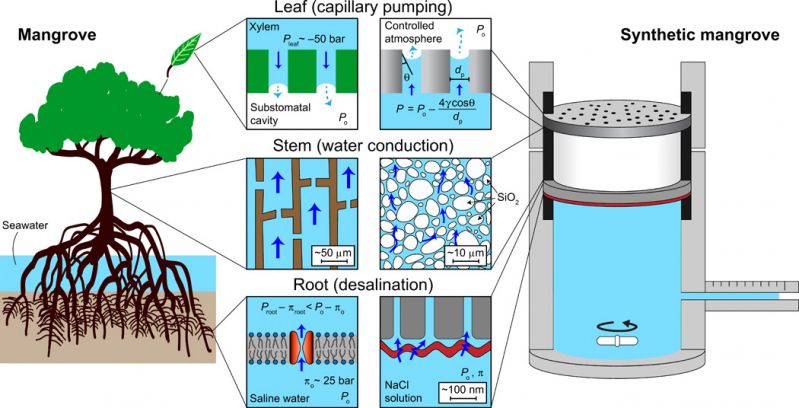
Taking inspiration from the distinctive properties of the mangrove tree, the research team was able to model desalination using their own artificial device. Though the device does not visually resemble a mangrove tree, it has parts that function similarly to the mangrove’s. Specifically, the device’s “leaves” undergo capillary pumping, the “stem” supports stable water conduction and the “root” aids in water desalination.
The mangrove desalinates water by using evaporation at the leaves to generate a negative pressure, which is felt by the water that enters the stem. This poses a problem, namely, that at negative pressures, water is metastable — it is capable of becoming a gas. Mangroves have special features that allow them to efficiently transport water without it turning into a gas.
The team faced challenges in creating a device that transported water without forming air bubbles. Bubbles create unwanted air pockets and reduce the efficiency of water flow. The team ultimately decided to use glass frit material, which has similar properties to that of the mangrove, to suppress bubble formation.
“We fabricated and optimized at least three devices,” Wang said. “In our device, the leaf, stem and root touch tightly to reduce water transfer distance and bubble formation possibility.”
The research team reproduced the large, negative, capillary pressures using pores that are strongly attracted to water. According to Werber, the device can generate up to -300 atm of negative pressure, the largest in an engineered device to date.
The Yale group also looked to confirm whether mangroves use the “cohesion-tension theory” to transport water.
Capillary-driven desalination in a synthetic mangrove
Abstract
According to the cohesion-tension theory, mangrove trees desalinate salty water using highly negative pressure (or tension) that is generated by evaporative capillary forces in mangrove leaves. Here, we demonstrate a synthetic mangrove that mimics the main features of the natural mangrove: capillary pumping (leaves), stable water conduction in highly metastable states (stem), and membrane desalination (root). When using nanoporous membranes as leaves, the maximum osmotic pressures of saline feeds (10 to 30 bar) allowing pure water uptake precisely correspond to expected capillary pressures based on the Young-Laplace equation. Hydrogel-based leaves allow for stable operation and desalination of hypersaline solutions with osmotic pressures approaching 400 bar, fivefold greater than the pressure limits of conventional reverse osmosis. Our findings support the applicability of the cohesion-tension theory to desalination in mangroves, provide a new platform to study plant hydraulics, and create possibilities for engineered membrane separations using large, passively generated capillary pressures.
INTRODUCTION
Mangroves are salt-tolerant trees that grow in tropical and subtropical coastal regions around the world. To survive in their saline or brackish environments, mangroves tightly control water and ion uptake at the roots, enabling xylem saps to be nearly salt free ( 1 – 3 ). Salt exclusion is partially achieved by physical blockage of the nonselective apoplastic route, in which water and ions circumvent the cell membrane by moving through the cell walls of root cells ( 1 , 4 ). This blockage stems from the deposition of a waxy substance called suberin within the cell walls of endodermal cells, rendering the otherwise permeable cell walls essentially impermeable. Elimination of the apoplastic route results in uptake occurring primarily via the symplastic pathway, in which water and ions permeate the cell membrane and subsequently pass cell to cell through intracellular cytosol until entering the xylem for long-range transport. The cell membrane acts as the discriminating barrier. Ions are largely excluded from passive transport through the lipid bilayer, owing to the high solvation energy of ions in the hydrophobic core ( 5 ). Ions must therefore pass through selective pumps and channels, allowing the cell to control ion uptake. Water readily permeates the cell membrane, with the permeability of the membrane being enhanced by salt-excluding water channels called aquaporins ( 6 ). Owing to the selectivity of the cell membrane, salt exclusion in mangroves can reach up to 99% ( 1 – 3 , 7 ).
Water permeates the cell membrane by moving down its chemical potential gradient, which in mangrove trees is affected by salinity (osmotic pressure) and hydraulic pressure. That is, selective water transport through the cell membranes of mangrove roots is a form of reverse osmosis (RO), the main technology used industrially for desalination ( 8 , 9 ). As in industrial RO, water uptake at mangrove roots requires a hydraulic pressure difference greater than the osmotic pressure of the saline water in soil pores (e.g., seawater with an osmotic pressure of ~25 bar). Since the hydraulic pressure in soil has a maximum of ~1 bar (ambient pressure), mangrove trees must exert a negative pressure within the root cells, which has been observed experimentally for mangroves and other plants ( 10 – 13 ).
The cohesion-tension theory is well accepted to explain the mechanism of negative pressure generation in plants ( 13 , 14 ). An air-water interface (meniscus) is formed in channels within the cell walls of leaf mesophyll cells, equivalent to nanopores with diameters of O (10 nm) ( 15 ). With the meniscus at a relatively stationary position, water molecules removed by evaporation are replaced by water flow toward the meniscus. The cohesive force between water molecules ensures continuous water supply, which in turn generates a tension (negative pressure) between the molecules. The water surface tension of menisci formed in nanopores with a diameter of 20 nm can generate a negative pressure of −150 bar in xylems, root cells, and leaf cells ( 14 , 16 ). Such highly negative capillary pressures serve as the primary driving force for water transport in plants, for example, enabling water to reach ~100 m heights in giant sequoia trees ( 14 , 17 ).
Plants use the capillary pressure–driven mechanism to transport water over large distances and heights with minimal expenditure of chemical energy. The main energy input is instead passive heat absorption from the environment at the sites of evaporation. Inspired by this ability, exploitation of negative capillary pressures has recently been explored to achieve passive water transport in engineering applications, including ion chromatography, microfluidic pumping, and chemical concentration ( 18 – 24 ). These engineered devices have also allowed for controlled experiments to better understand complex plant hydraulics, for which much is still unknown ( 25 , 26 ). A major complication in plant hydraulics and in these engineered devices is the metastability of water under tension, as metastable water is prone to the formation of bubbles (cavitation) ( 16 , 25 – 27 ). For mangroves, the requirement of highly negative pressures to overcome the osmotic pressure of the surrounding saline water substantially increases the vulnerability to cavitation. Plants, including mangroves, have developed highly intricate structures to minimize cavitation and prevent embolism (blockage) of the xylem ( 1 , 13 , 28 ). In engineered systems, the challenge of maintaining bubble-free conduits has thus far limited capillary-driven fluidic transport to relatively low-pressure applications, typically less than 25 bar (the osmotic pressure of seawater). Furthermore, although mangrove-inspired membrane materials have been created ( 23 , 29 ), the negative-pressure–driven desalination of brackish and saline waters in mangroves, per the cohesion-tension theory, has not been mimicked in a synthetic system. Demonstration of large, stable negative pressure in a synthetic system is needed to expand the potential use of capillary pressures to high-pressure engineering applications such as desalination, chemical purification, or chemical sensing.
In this work, we present a synthetic mangrove that captures the main features of natural mangrove trees: capillary pumping (leaves), water conduction from root to leaves in a highly metastable state (stem), and desalination (root). Specifically, the synthetic mangrove consists of a nanoporous anodized aluminum oxide (AAO) membrane or a poly(hydroxyethyl methacrylate)–based hydrogel as leaves, a porous fritted silica filter as the stem, and a polymeric RO membrane as the root. We demonstrate that the synthetic leaves can generate highly negative pressures that enable desalination through the RO membrane. Even under such highly metastable states, the synthetic mangrove maintains stable water uptake and desalination of saline water. Our work supports the cohesion-tension theory for desalination in mangroves and highlights the potential to use highly negative capillary pressures for diverse engineering applications.
RESULTS
Working principles of the synthetic mangrove
Design of the synthetic mangrove device sought to capture the essential features of transpiration and desalination in the mangrove tree. The main components are illustrated in Fig. 1 and fig. S1, with their natural analogs in the mangrove tree displayed as well. Desalination occurs at the root, which in the mangrove tree is the aquaporin-containing cell membrane. The synthetic mangrove uses a commercial, polymeric RO membrane that is designed for seawater desalination (SW30HR, Dow Water & Process Solutions). This membrane comprises a thin (
Media
Taxonomy
- Desalination
2 Comments
-
Good job.
Regards
-
Interesting development