Arsenic removal from the water technologies
Published on by Karan Kurien, KK environment - Technical director in Technology
Hello everyone, we are having Arsenic contaminated water on our project site. Can anyone explain how to distill it out of water?
Taxonomy
- Water
- Public Health
- Arsenic
- Environmental Health
- Purification
- Arsenic Mitigation
- Water Purification
22 Answers
-
Dear sir,
we require more information like: quantity of water to be treated. total dissolved solids of the water, pH of the water. We can give you chemical precipitation method with treatment scheme include Lamella settler, MGF, ACF, resin filtration+ RO. the treatment scheme depend on concentration of nickel and other heavy metal in water.
please share analysis report of the concern water, so we can give you detail techno-commercial offer.
Thanks
vimalesh Patel
Head Operation
Jyoti Hydrotech private Limited
+91 9327116113
email: vimalesh@jyotihydrotech.com, info@jyotihydrotech.com
1 Comment
-
is there a tech writeup/spec of the precipitation method that i can get? regards
-
-
Dear ALL
We have Arsenic (50-250 ppb) in some areas where hand pump is the only way of getting potable water. Can anyone help me find a cartridge that my people can fit at the delivery point of the hand pump and get an Arsenic Free Water?
-
Hi Karan,
I can offer a sustainable method of Bioremediation to remove Arsenic and with this not just arsenic is removed but you are able to make some money as well selling off the metal.So a two-in-one project.
1 Comment
-
Is there any spec for the method you offer? and the cost? and would it work in developing countries where power availability may not be sustained? thanks
-
-
Prompt whatvolume ofcontaminated wateryou needto clean???
-
Please note that the most cost effective technology to do this with is to use Bayoxide adsortion product. Pleawse contact me if you would like to have more specific information
-
I already wrote aboutthe fact thatby applyingour equipment, you canget a cleanprocess water.We cancarry outtestson ourtest bench,if you want.
-
The use of (MgO) Magnesium Oxide will take of this problem permanently. It places a positive charge into the water that shatters the magnetic bond of any pollutant or contaminant and gasses it out of the water, raises the pH to 8.7, high alkalinity, high oxygen and the water is structured thinner. The MgO placed in nylon bags simply placed in the bottom of your tank will permanently remove arsenic, pesticides, pharmaceuticals, radiation, heavy metals, CHROMIUM-VI, lead and any other totally dissolved solids. It will never wear out, never dissolve and never need to be replaced.
-
Arsenic is similar to a heavy metal, therefore, it can be eliminated with chelating ion exchange resins

1 Comment
-
This Post is a dialogue of the deaf because no one comments on the interventions of others. But I would add that the wells do not only contain arsenic but also nitrates and pesticides. The water oxidation treatment against this pollution can be done right in the wells, too, producing cheap energy. I look for international partners for both solutions.
The invention of the pump with two separate supply until the impeller on the suction side has allowed the invention of hydropower by recycling water in an open vessel. With this system we have, at the same time, lifting water and the production of energy, mainly by exploiting the dynamic pressure (or kinetic energy) of the water flowing from the upper reservoir.
This invention, in turn, has inspired the present invention, which interacts positively with the environment. In fact, in pressurized systems with pumps with double separate power supply, the continuous internal recycle to the volume of water accumulated allows balance the hydrostatic pressure in inlet and outlet of the pump and therefore to circulate the water with very little energy.
With the second separate supply until the inside of the impeller, it is possible to introduce water at low pressure in the pressurized tank. Since water cannot be compressed, the same amount is excreted in high pressure by the cushion of air through a tube that feeds a turbine, transforming the energy of static pressure into dynamic and producing electricity. The compressed air volume does not vary, therefore the air behaves like a spring, but due to of the principle of Dalton on the partial pressures of the gases and Henry on the solubilization of the gases, neglecting the effect of nitrogen that is neutral and of the other gases that are in negligible percentages, we have an important effect of the oxygen solubilization due to the pressure which increases proportionally according of the same. This involves an increase in capacity of water purifying, without increasing the cost of production of electricity. Considering that to state of the art pressurized water power does not exist, it is clear that this invention involves huge economic and environmental benefits. In this application we can make small vertical submerged pressurized water power plants in wells polluted by nitrates and pesticides that purify the groundwater while producing energy. But the same application can be produced in a reduced version even in small treatment plants. All the hydraulic systems, including those depurative, of the future will be able to produce energy because the power of compressed air always allows to have residual energy to be exploited in a turbine and all will be with the water recycling, because the recycling of water by the pumps with the dual separate supply allows to circumvent the force of gravity and the pressure. The compressed air will be used as an accumulator of energy that disperses only the part of the energy due to the components that dissolve in the water chemically. This dispersion in global systems, which are also depurative, cannot be considered a loss of yield. With this invention we move towards the elimination of energy costs and multiplies environmental protection.
-
-
Hi Karan. To study the appropriate solution to contamination of water with arsenic need to know more data source, such as:
Level of concentration of As III and As V (Arsenic speciation as AsH3O3 - AsHO4-2).
Production flow or treatment.
pH
Phosphate concentration.
Silicate concentration.
Iron concentration.
Then these data you can evaluate the level of competence of other chemical species for arsenic and see which treatment to apply. Adsorption is particularly very suitable for its high retention and low environmental impact process.
Currently there are many adsorption resins based oxides do or granular ferric hydroxides, as with MnO2 structures.Good luck.
Claudio
-
Depending on your setting, naturally occurring or non-point sources. I would suggest alum, this will drop out or precipitate most of the TDS and may be any easy fix. Though, pH/Eh would be certainly relevant. R/O would work but is prohibitive in cost. Looking at the water's source would be highly advisable. I'm not sold on the biological approach but there are many methods once again depening on your setting, usage, flow rate and other variables.
check out http://www.sswm.info/content/arsenic-removal-technologies you also might want to check out specific conductance and dissolved oxygen levels. Is the source water, surface groundwater, anaerobic or not.
good luck
bil
-
In this video removal techniques are explained from the Tagore-SenGupta Foundation in Cambodia based on adsorbants. Also they explain that besides offering arsenic free water, efforts are invested in promoting this new water source as people are (obviously) used to their own source close their homes. www.thewaterchannel.tv/media-gallery/5888-video-winner-of-2011-environmental-challenge-installs-arsenic-groundwater-removal-system-in-cambodi
-
In this video removal techniques are explained from the Tagore-SenGupta Foundation in Cambodia based on adsorbants. Also they explain that besides offering arsenic free water, efforts are invested in promoting this new water source as people are (obviously) used to their own source close their homes. www.thewaterchannel.tv/media-gallery/5888-video-winner-of-2011-environmental-challenge-installs-arsenic-groundwater-removal-system-in-cambodi
-
There are several different treatment technologies available for the removal of arsenic from polluted water bodies. However, I would suggest the bacterial treatment would be one of the promising methods for arsenic removal.
Since, bacteria are active scavengers of metal ions and have larger surface area to volume ratio, the direct adsorption of arsenic to the charged surfaces of the bacterial cells could occur, secondly the potential of bacteria to oxidize Ar(III) to Ar(V) could also be utilized in the treatment process, third bacteria promotes the oxidation of iron. The so formed biogenic oxides of iron could accelerate the adsorption of arsenic. However, it is cautioned that bacteria could also convert a less toxic element to a more toxic form and viz versa depending upon the environmental conditions.
You may refer to the below links and to the earlier reply from other colleagues.
http://www.sswm.info/content/arsenic-removal-technologies
http://www.sciencedirect.com/science/article/pii/S0959652612000492
-
Morning Karan
Arsenic can be effectively removed by using Lanxess' Lewatit ion exchange resin or Bayoxide adsorbent medium. Please provide your feed water analysis (pH, Eh included) as well as the specifications for the final water quality, as well as the water flowrate. Lanxess can then provide you with information on what the most cost effective option would be for you for this treatment.
Marthie Kotze
-
hello Karan...... There are Arcenic removal medias available for more on it write to me on aquariusent@gmail.com ,
-
Large orconstant volume ofwaste waterwith arsenic??
IfDC-resetmyanalysis of waterandwe are ready toofferyou the equipment.tumentsev@inbox.ru
-
I agree with the answer by Aseem Murtaza. For arsenic removal from drinking water, ground water etc. metal hydroxide (e.g. ferric hydoxide) based filter materials are used oftenly. Sometimes oxidation is necessary as pre-treatment, especially when a big part of the arsenic to be removed is 3-valent arsenic.
I disagree with the main article that adsorption is the only process which doesn´t need a pre-treatment. Adsorption needs always a pre-filtration, no matter if the adsorption material is activated carbon, ion exchanger resin or metal hydroxide.
-
Arsenic, As, is a chemical element that is found in many minerals, often in combination with sulfur and metals, but also as a crystal.
It is odorless and tasteless. It can enter drinking water supplies from natural deposits in the earth or from agricultural and industrial practices.
Effect of Arsenic on human health: Long-term exposure to inorganic arsenic, mainly through drinking of contaminated water, eating of food prepared with this water and eating food irrigated with arsenic-rich water, can lead to chronic arsenic poisoning. Skin lesions and skin cancer are the most characteristic effects. Non-cancer effects of arsenic can include:
- Thickening and discoloration of the skin,
- Stomach pain,
- Nausea,
- Vomiting,
- Diarrhea,
- Numbness in hands and feet,
- Partial paralysis, and
- Blindness.
Permissible level of Arsenic in drinking water: It should be 10 µg As/l according to WHO and EPA standards.
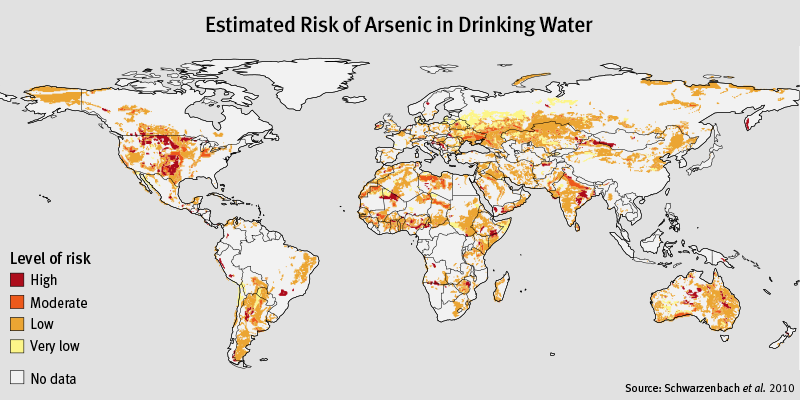
Countries affected by Arsenic contamination: Inorganic arsenic at high levels in ground water can be found in Argentina, Bangladesh, Chile, China, India, Mexico, and the United States of America.
Bangladesh is one of the most affected countries by arsenic. In the 1990s arsenic was widely present in well-waters in Bangladesh. The standard in Bangladesh is 50 µg As/l compared to the WHO and EPA standard of 10 µg As/l in the drinking water.
Technologies for Arsenic removal from water:
- Coagulation, precipitation and filtration
- membranes
- sorbent processes
- adsorption
- ion exchange (strong base resins)
- oxidation
- Alternative methods
1. Coagulation, precipitation and filtration
Coagulation
Coagulation is a water treatment process in which chemicals - coagulants are added to the water – a colloidal suspension.
In colloidal suspensions the particles would settle very slowly or not at all, since they carry surface electrical charges. This causes the particles to repel each other. In order to overcome that coagulants are added.
Coagulants can be Aluminium salts, Iron salts, Iron hydroxides, lime, etc.
Coagulation with Aluminium- and Iron-based coagulants, followed by disinfection by chlorinating is one of the most used methods of water treatment.
Water pretreatment by oxidation might be necessary.
When the coagulants are added to water and stirred for a few minutes they dissolve.
Flocculation is a process in which the colloids from a suspension end up in flocs of flakes.
During flocculation, due to the electrostatic attachment, the micro-particles and negatively charged ions get attached to flocs. Arsenic is adsorbed onto the coagulated flocs.
Flocs can further be removed by sedimentation, but only partially and filtration may be necessary for complete removal.
Pipe flocculation followed by filtration with sand filters is >99% efficient in removing Arsenic.
Precipitation
Lime is a white inorganic material consisting of calcium oxide. Arsenic can be removed by adding quick lime – CaO or hydrated lime – Ca(OH)2.
Lime treatment is similar process to coagulation with metal salts. Some of the lime will dissolve and the arsenic will attach to Calcium hydroxide - Ca(OH)2 since Ca(OH)2 acts as a sorbing flocculent.
Excess lime doesn’t dissolve and needs to be removed later by sedimentation and precipitation processes.
2. Membranes
Membrane is a selective barrier – it lets some things pass, depending on the membrane, and is an obstacle to others.
The filtering occurs because the tiny holes in the membrane are large enough to let the water pass but small enough to block the undesirable materials.
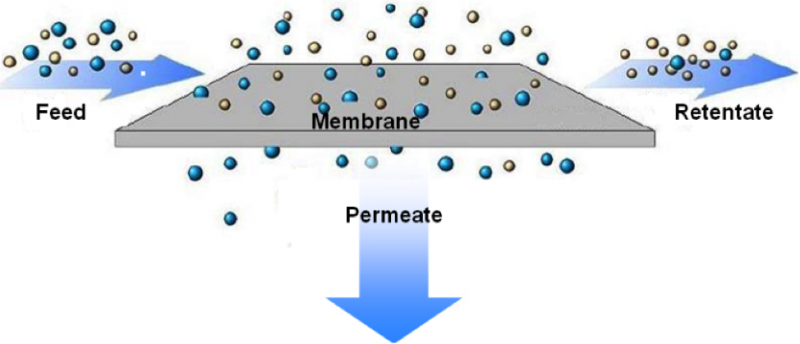
Diagram 1: Membrane process
Diagram Source: Journal of Organic Chemistry*
Synthetic membranes remove contaminants from water including pathogens, salts and various metal ions.
Two types of membrane filtration are used, depending on the water pressure needed for the filtration:
- low-pressure membranes such as micro-filtration and ultrafiltration
- high-pressure membranes such as nano-filtration and reverse osmosis.
Nano-filtration is a membrane process with the holes in the nanometer range. It is usually used when total dissolved solids are low.
3. Sorptive Filtration
Sobrent is a material used to absorb or adsorb liquids or gasses. It has the property of collecting molecules of another substance by sorption.
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a fluid to a surface. A film of the adsorbent is created on the surface of the adsorbent. Adsorption is a surface-based process while absorption involves the whole volume of the material.
For Arsenic removal the adsorbents used are:
-active carbon
- zeolite, hydrated metal oxides (active aluminium trioxide)
- ion changing resins
- kaolinite compositions and humane acids
- porous resins with crystal hydrated zirconium oxide, etc.
Adsorption is the only process for As removal that does not need the water pretreatment!
Ion exchange
Ion exchange is a physical-chemical process in which ions are swapped between a solution phase and solid resin phase. If As(III) is present, it must be oxidized to As(V) in order for ion exchange to be effective.
Ion exchange resins are ion exchange polymers that are insoluble. They form small beads that are porous (water can pass through them). That way they provide a large surface area.
Ion exchange is similar to that of activated alumina. The difference is that the medium is a synthetic resin that has better-defined ion exchange capacity.
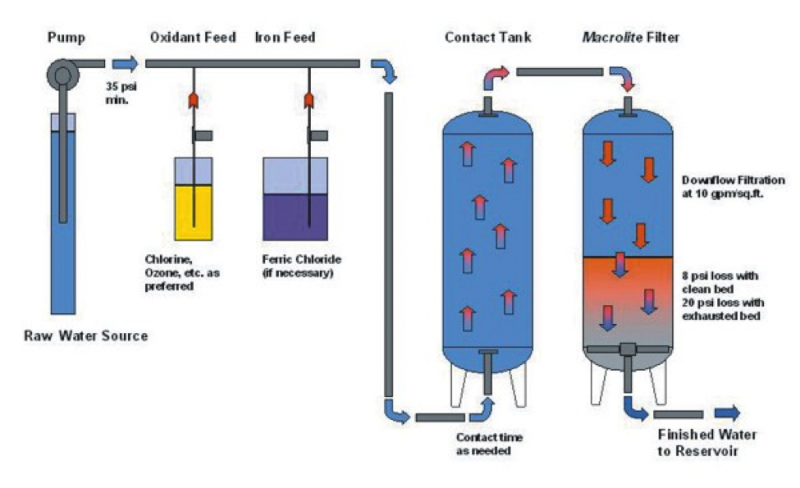
Diagram 2: Ion exchange Arsenic removal
Diagram Source: Environmental Science & Engineering *The process is less effective if the concentration of dissolved matter is high.
4. Oxidation
Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Oxidation is need for As(III) removal, since it is much easier to remove As(V) than As(III).
At pH 6.5 to 8.5, As(III) is present as uncharged arsenious acid - H3AsO3 under reducing conditions, whereas As(V) is present in the form of singly and doubly charged H2AsO4- and HAsO42- anions in oxidizing waters. Thus, processes that remove anions are suitable for removing As V but not As III.
Atmospheric oxygen is the most available. However, air oxidation of arsenic is a very slow process and can take weeks for oxidation. Air oxidation of arsenite can be catalysed by bacteria, strong acidic or alkali solutions, copper, powdered activated carbon and high temperature.
UV photo-oxidation gives promising results converting As(III) to As(V) when sulfates are added. Otherwise it is inefficient.
Efficient oxidation is achieved with Chlor, permanganate and ozone.
Their ability to convert As(III) to As(V) is relatively not affected by the water pH, in the pH range of 6.3 - 8.3.
5. Some other alternative methods
There are some alternative methods like SORAS, Solar Oxidation and Precipitation of Fe, Phytoremediation like the use green plants to remove pollutants from the environment etc.
References:
Groundwater Arsenic Contamination Problems in Bihar: Causes, Issues and Challenges
STUDY OF VARIOUS METHODS FOR THE REMOVAL OF ARSENIC CONTAMINATION IN GROUNDWATER
Design of cost efficient filtration cartridge for the removal of arsenic and iron
Arsenic and Iron Contamination in Ground Water in Lower Bramhaputra Basin.
Articles published on TWN about Arsenic can be seen here.
1 Comment
-
Interestingly, above world map and legend show "No data" for Bangladesh, while in 2002 the WHO named Bangladesh as "The largest mass poisoning [by Arsenic] of a population in history" ... http://www.who.int/features/archives/feature206/en/
Most of above mentioned As removal technologies are too expensive and too high-tech for rural Bangladesh.
SORAS (Solar Oxidation and Removal of Arsenic by Wegelin et al.; http://wedc.lboro.ac.uk/resources/conference/26/wegelin-soras.pdf) as also mentioned at the end of above post under "5. Some other alternative methods", at one time appeared as a promising solution for As removal in developing countries such as Bangladesh. However, I don't know, if it ever became a break-through method like SODIS (Solar Disinfection of Water; www.sodis.ch) did.
-
Removal of Heavy Metal from neutralized waste derived from waste water, the heavy metals present in the neutralized waste water , will be removed by chemical precipitation with Sodium Sulphide. Chemical precipitation of heavy metals like Arsenic, Copper, Chromium, Nickel, Zinc & Vanadium by Sodium sulphide under alkaline condition
2 As3+ + 3 Na2S = As2S3 + 6 Na+
-
arsenic removal
The current drinking water standard is 0.010 mg/L or parts per million (ppm) for Arsenic. This is equivalent to 10 ug/L (micrograms per liter) or 10 ppb. Long term exposure to drinking water containing arsenic at levels higher than 10 ppb increases the chances of getting cancer, while for lower arsenic water levels the chances are less But if your arsenic level is upto 500 ppb , showering, bathing and other household uses are safe. Arsenic is not easily absorbed through the skin and does not evaporate into the air. So water treatment for arsenic removal also depend on your use.
Arsenic removal methods or systems include anion exchange, reverse osmosis, activated alumina, and other types of adsorptive media filters. Each method has its advantages and disadvantages.
Some studies are carried out for arsenic removal, refer to Cost Effective Method for Removing Arsenic from Water at http://www.lbl.gov/tt/techs/lbnl1742.html.
Also refer this link for further details, http://www.water.siemens.com/en/applications/drinking_water_treatment/arsenic-removal/Pages/default.aspx
As said each method has ist advantages as well disadvantages. Thanks, cheers…
-
Arsenic contamination of drinking water is a global problem that will likely become more bigger in future. The primary methods for removing arsenic from drinking waters include Ion exchange using a resin to remove anionic As species, Coagulation/Micro-filtration Adding Fe(III) or Al(III) salts to form arsenic-sorbin flocs which are subsequently removed from solution by granular media or membrane filtration, Fixed bed adsorption Removal of arsenic with an adsorbent, typically a metal (hydr)oxide such as ferric hydroxide or AA (activated alumina), Lime-softening Adding lime to soften water (remove Ca and Mg) often removing appreciable amounts of arsenic in the process through sorptive uptake by metal carbonates and hydroxides, Iron removal Oxidizing reduced iron to remove arsenic through sorption/coprecipitation/coagulation, • Physical filtration To remove colloidally-bound arsenic, Membrane processes Membrane removal of arsenic by Reverse Osmosis (RO) or Nano filtration (NF)
-
Arsenic removal comments already posted
Hi Karen,
Following are some links already posted on The Water Network addressing this question. We will forward to others to get additional input from the membership.