What do you know about coagulation? Can it be applied to cleanse turbid water?
Published on by Hussein Ibrahim, Previous Eastern Nile Watershed Development and Management - General Agricultural Specialist in Technology
Most of the rural areas in the “Developing countries” depend on surface water such as irrigation channels or open ponds for their drinking and other domestic uses. Towards the end of the cultivation season, the water supply in the irrigation channels is occluded, the water then becomes shallow and turbid, and by the end of rainy season the remaining water in the surface which collected in surface depressions (open ponds) becomes depleted by vaporization. Both water sources ultimately become turbid and contaminated, which constitutes a hazard for rural inhabitants.
This warrants upgrading water quality through purification, decreases of turbidity, and removal of pathogens.
Turbidity
The turbidity of surface water is increased when rain water runs across the surface of the ground, forming open pools in surface depressions. The rain water picks up many substances such as suspension materials, collided materials, minerals, salts, organic and inorganic materials. This provides a perfect medium and adsorption site for chemicals, causing organic and biological reactions which result in aesthetically undesirable tastes and odor. Also the turbid water provides a perfect medium for the growth of all type of microorganisms and pathogens which are responsible for causing the people the majority of diseases and infection.
Taxonomy
- Pathogen Removal
- Bacteria
- Coagulants
- Purification
- Filtration
- Water Purification
16 Answers
-
Coagulation and flocculation processes are used to facilitate the elimination of suspended solids and colloids by gathering these together to form the floc. Sedimentation process, flotation and/or filtration systems are used after coagulation and flocculation to separate the floc.
Factors that influence destabilisation of colloidal particles are type of coagulant added to water and velocity gradient, G. In order to be effective, coagulant must be dispersed immediately and therefore optimum G factors range from 400 – 500 s-1 and up to 1000 s-1.
Velocity gradient is determined by equation
G =

Where
G= mean velocity gradient (s-1)
P- power actually dissipated, W
V - fluid volume, m3
µ - dynamic viscosity (Pa s)
Optimum retention time is from 30 – 60 s.
After particles neutralization and agglomeration of particles, floc is formed.
The optimum G values in flocculation should be up to 100 s-1. It is advisable to have at least 3 stages of flocculation, where the G value will be lowered from I stage to III stage to (20 – 30 s-1.). To further improve the flocculation process, it is good to have variable speed mixers with possibility to change G value. Special types of mixers are needed for good flocculation process.
The overall retention time in all stages of flocculation should be not less then 20 min but could be longer (up to 30 minutes).
Regarding the coagulants, one of the most applied coagulant is alum sulpahte, which is manufactured in Saudi Arabia so there will not be much problem to use it.
As for the flocculation, various types of polyelectolytes are available.
There are some products which present combination, so called aluminium polymers PAC, PACS, etc.
In addition, pH of water plays significant role in coagulation and flocculation process. For optimum consumption of alum salts it should be in the range of 6.0 – 7.2 in coagulation process.
-
The Water Network research team has consolidated an answer to the above question.
Further input from members is welcome and appreciated.
Coagulation is a water treatment process in which chemicals (coagulants) are added to the water.
It neutralises charges and forms a gelatinous mass to trap particles, forming a large mass that settles or is trapped in the filter.
Dissolved and suspended particles can be found in most of natural waters. These suspended materials mostly come from land erosion, dissolution of minerals and decay of vegetation and domestic and industrial waste discharges.
The water quality is deteriorated with their presence so they have to be removed.
Coagulation and flocculation are relatively simple and cost-effective, provided that chemicals are available and dosage is adapted to the water composition.
In colloidal suspensions the particles would settle very slowly or not at all, since they carry surface charge. Same charges repel each other. Coagulants are added in order to agglomerate the particles.
Coagulants can be classified into:
· inorganic coagulants (e.g., aluminum and ferric salts)
· synthetic organic polymers (e.g., polyacryl amide derivatives and polyethylene imine)
· natural coagulants .
All of them are very efficient at turbidity removal from water.
Coagulation with Aluminium- and Ferric-based coagulants, followed by disinfection by chlorinating is one of the most used methods of water treatment.
Water pretreatment by oxidation might be necessary when using them.
When the coagulants are added to water and stirred for a few minutes they dissolve.
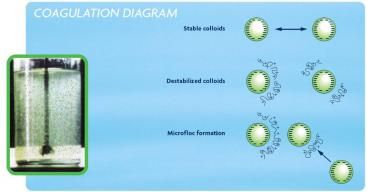
Diagram 1: Coagulation
Source: sswm.info/content/coagulation-flocculation
Coagulation destabilises the charge of the particles.
Coagulants with charges opposite to those of the suspended solids are added to the water to neutralise the charges on dispersed non-settable solids such as clay and organic substances.
Once the charge is neutralised, the small-suspended particles stick together.
The slightly larger particles formed through this process are called microflocs and are invisible to the naked eye.
To achieve good coagulation and formation of the microflocs, a high-energy, rapid-mix to properly disperse the coagulant and promote particle collisions is needed.
Over-mixing does not affect coagulation, but insufficient mixing will leave this step incomplete. Proper contact time in the rapid-mix chamber is typically 1 to 3 minutes.
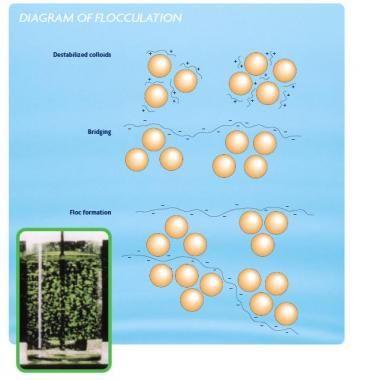
Diagram 2: Flocculation
Source: sswm.info/content/coagulation-flocculation
Flocculation is a process in which the colloids from a suspension form flocs of flakes.
During flocculation, the micro-particles and charged ions are attracted and attached to flocs. The impurities are adsorbed onto the coagulated flocs.
Flocs can further be removed by sedimentation, but only partially and for complete removal filtration may be necessary.
Gentle mixing stage, increases the size of submicroscopic microflocs to larger suspended particles.
With slow mixing, the microflocs are come into contact with each other. Collisions of the microfloc particles cause them to bond to produce larger, visible flocs. The floc size continues to grow through collisions and macroflocs are formed.
Separation is done (sedimentation, floatation or filtration) when the floc has reached its optimum size and strength.
Contact time for flocculation ranges from 15 or 20 minutes to an hour or more.
Since the question is focused on water purification in rural areas, we are going to mention some of the natural coagulants and especially focus on Moringa Oleifera which is easily available in Saudi Arabia where the question author is from.
Some of the natural coagulants are:
· Moringa Oleifera
· Strychnos potatorum, also known as clearing nuts or the nirmali tree, found in India
· Prickly pear cactus, traditionally used in Latin America.
· Fava beans.
 Moringa Oleifera
Moringa Oleifera Natural coagulants are differently available depending on the region, which is both an advantage and a disadvantage.
The Moringa Oleifera tree grows in tropical and subtropical regions.
It needs six months to reach fruition and is used in many areas as a food source. The seedpods, seeds, leaves, roots and flowers are all edible and nutritious.
Moringa Oleifera trees are widely present in Africa, the Middle East and the Indian subcontinent.
It is a fast growing (4.6 meters/year) crop tree that does well in the equator belt climates with non-bogg y soils.
The tree's seed grow in pods. Dried, hulled, and ground seeds have a significant ability to reduce the suspended solids in extremely turbid waters.
Dried beans and seeds can be stored for a long time.
The shelled seeds provide much higher turbidity removal than the non-shelled ones.
Moringa Oleifera is a coagulant and an antimicrobial agent.
When adsorbed onto sand, a protein in dried and ground seeds, has excellent results in removing total suspended solids (TSS) and killing coliform bacteria.

A positively-charged protein called the Moringa Oleifera Cationic Protein (MOCP) is responsible for the excellent water-clarifying ability of the seeds.
When the seeds are crushed and added to water, this protein will kill the microbial organisms and cause them to clump together and settle at the bottom.
Moringa Oleifera seedsalso have the ability to reduce E. Coli.
* A dominant mechanism of MOCP antimicrobial activity is membrane fusion. Cryogenic electron experiments on E. coli cells established that MOCP fuses the inner and outer membranes.
Bacteria and viruses can attach themselves to the suspended particles in water and cause turbidity. Therefore, reducing turbidity levels through coagulation may improve the microbiological quality of water.
The dried seed powder alone is not ideal for water purification because the organic matter from the seed will remain in the water, providing a food source for any bacteria that have not been killed. As a result, water treated with this seed does not remain safe to drink after some time in storage.
Water clarified with Moringa seeds must be used immediately.
Coagulant Optimum dose (mg/l) Turbidity removal (%) DOC change M. oleifera seeds 100 – 64% reduction M. oleifera seeds 50 90 >50% increase M. oleifera seeds 6000 - 52% reduction 1-Step purification of M. oleifera 2 97 32% increase 2-Step purification of M. oleifera 2 98.5 17% increase Table 1: Turbidity removal and DOC change depending on the Moringa seeds dose
Source: sciencedirect.com/science/article/pii/S2212371714000171
Documents on TWN about Coagulation and Moringa Olifera: -
crowdsource challenges and solutions via the Marketplace
You may find solutions to your challenge or even post this challenge and get solutions via an exclusive infrastructure community, the Marketplace of the Innovation Interchange https://www.innovationinterchange.com/products/marketplace
When facing intractable challenges, owners and operators of infrastructure assets can find solutions from across the globe via the technology Marketplace. Register free online and connect with other organizations as you crowd source challenges and solutions.
-
Dear All Thanks for your answers and Comments ,,, In some of the Rural Areas the people use some natural substance and not chemical for the Coagulation more safety , this natural materials is used to be taken from trees and it is effective ,, such as Moringha Tree ,we don't know what is the mechanism , but it helps in separating and remove the turbidity BR, Hussein
-
Dear All Thanks for your answers and Comments ,,,Then I think that we need primary stilling Tanks for the coagulation process by adding some chemical that accelerate the accumulation of slitting eg. and then connected this tank with Re-washable Sand Filter for more purification , we need some such means in Rural Areas , BR, Hussein
-
Coagulation is designed to surround small particulates and get them to settle out.
Coagulation is basically designed to surround small particulates and get them to settle out. Therefore, the expense is a major concern to this type of process. That is the main reason that a series of settling tanks may be a better solution? We can usually figure this out with normal water and wastewater calculations...
-
Prof Mohamed Habib Sellami
The coagulation theory and its application to treat the turbilidity of surface water particularly for rural area
The surfce water are generally in flowing with relatively more or less important velocity so the particules that exist are in displacement with the same speed and in the same direction. If we apply coagulants in the purpose to accumulate particules together in flocs with high size we risk to not reach that objectif because of the flowing or we must choose a coagulent with high density so it resist to the flow but in this case there's problems of cost and the impact of that coagulant.
Perhaps we have to construct many tanks on the traject of the channel so we can retain the water for some time (to define) and we add the coagulant in the tanks so we assume the optimal conditions of the treatment with coagulation technique
-
Coagulation & Disinfection
Dear Mr. Hussein Ibrahim,
Your question carries basically two concerns
- Turbidity
- Pathogens
Turbidity , could easily be catered by any suitable coagulant such as Alum mostly used in rural areas and is commonly used by them as well for such purpose for settling the suspended particles. This could be done even for ponds, shallow channels as well. Alum is very effective for removal/settling quickly the turbidity.
Pathogens/Bacteria
For this, commonly and cheapest disinfectant is NaOCl , Sodium Hypo Chlorite. Easily available in market even in rural areas and most cheapest one and very effective disinfectant.
The above two options could be carried out in rural areas for a safer drinking water in a broader spectrum. The dosage of both i.e. Alum and NaOCl could easily be determined by the size of the pond or length (depth) of Channel.
Thank you
Best regards,
Engr. Mansoor Ahmed
PhD Scholar, ME, MSc Environmental Engineering
-
First is you should know about the characteristics of your turbid water( mosly pH only ) in order to know the type of flocculant that you will need(ionic or cationic flocculants). Flocculants makes all suspended solids to adhere to each other thus the coagulated solids will have a higher density than the water which will make it separate. You can separate/decant the solids by filtration or the other means. In some cases where suspended solids are minimal, a flocculant may not be necessary and an RO system can solve the issue. No chemicals needed but a physical separation only.
-
It is usually used to increase effectiveness of disinfection, and not separately. Without coagulation the disinfectant is much less effective. Have you investigated slow sand for a really really low tech application? It still requires an operator with understanding of the process, but requires no special chemicals to enhance the effectiveness. I would only use it when other options were unavailable, but it can work. Combine slow sand with leaving bottles in an ultraviolet source (sunlight) in bottles that are permiable to UV and I would feel pretty good about an improvement of two or three orders of magnitude. But always test your product!
-
Coagulation Theory has to do with electronic charges and attraction and repulsion of particles in suspension. It works because particles, during Brownian motion, become closer than a certain radius that determines that they will be attracted to each other. The number of collisions is related to the amount of turbidity. The concentration of particles in the effluent is what determines how safe the water is. If it is too high, the particles may either carry, or indicate the presence of, or inhibit the disinfection of protozoans and other biological agents. You can ask the question about whether it is effective in some water or another but, in the end, jar testing and sampling of the effluent is what determines effectiveness. There are polymers, cationic agents and anionic agents and even bentonite clay that I have seen work to improve the surface charge or particle density of the solution. Usually you want to try out Alum or ferric chloride for cheapness and some specialty chemical polymer for efficiency.
-
as I do not cease repeating it on the forums the question does not arise of knowing if one can do this or will clera with such or such device. 'turbid' water thus - soiled are they always BIOLOGICAL? All is the question there, because if they are not any more in 'biological' state, after any unspecified treatment, that they become? if they are in 'biological' matter it there no problem to undergo a treatment of biological purification. if they are impregnated micro pollutant chemical those Ci will be present at exit of any treatment. I am frightened that at our time the 'Biological' characteristic is forgotten at this point. After one can do everything but which will be the consequences about it. one proposes to me to make a treatment of the liquids 'lixitiat' of one THIS of class 2 of a volume of 500m3 day. If this liquid contains micro pollutant chemical, biology can nothing make. one proposes to me to reject the effluent in the river like that is currently done. it is already in the train of pollor in a perennial way the environment and one me porpose to continue when precisely I fight so that the ground serf more dustbin.
-
Coagulation and settling in irrigation channels
Coagulation usually involves addition of chemical products (coagulants such as ferric chloride, ferric sulfate and alum) which neutralize the charge of the naturally occurring particles in the water and attach these particles to the coagulant thereby forming larger particles that can easily settle in quiescent conditions. The problem with applying such coagulants directly to the irrigation channels is that the coagulated particles may not settle because of the relatively large flow through velocity in the channels. In order for the particles to settle well, the velocity in the channel should be less than 0.1 m/s, which may not be practical. In addition, the introduction of coagulant to the irrigation water will create significant amount of extra solids - for every 1 mg/l of coagulant approximately 0.8 mg/L of extra solids are created. So coagulant addition, even if the coagulated solids can settle, would fill up the irrigation channels after a while. In order to coagulate irrigation water it is recommended the coagulation process to be completed in a separate settling tank/pond where coagulant would be added. The clarified water exiting this tank will be used for irrigation and the solids retained in the tank will need to be removed for offsite disposal.
-
coagulation can remove color and suspended solids, but I'm not certain it can reliably purify water to safe drinking water quality for pathogens or contaminants other than hydrophobic ones. Additionally, the flocculant will still be present at the bottom of the water body treated. So any contaminants or pathogens will not really be removed from the system, just moved to another place in the system with the ability to be remobilized. Murky water is still useful for irrigation, and other safer techniques exists for rendering water potable.
-
coagulation
Coagulation:
~~Equipment/skill required:
A coagulant (ex. aluminium sulphate)
Some skill/training
2 vessels
Stirrer
Filter – a clothProcess:
Add a coagulant to water and stir rapidly.
Allow to stand for some time, and continuously stir slowly to form large flocs.
The formed flocs clean the water by attracting pathogens and other microorganisms.
Remove the flocs using filtration and be careful to not re-contaminate the water.~~Advantages:
Proven reduction of viruses, bacteria, protozoa
Pesticide and heavy metal removal
Simple technology and useDisadvantages:
May be toxic if used improperly
Could be more expensive and complicated than other methods due to increased number of required materials and skill
Requires multiple steps -
It seems to be a general question. Coagulation theory is a very complex issue to be answered shortly.It is normally aplied in surface waters and even in turbid waters. It is easier to deal with turbid waters, since the system has been well designed than coloured and low turbidity waters where coagulationj shall be applied correctly.