Low COD removal, what is the best for use as RO system feed?
Published on by Ahmad Bahlake in Technology
H everybody.
We want to reduce COD from 50 to 10 ppm. Also the feed consisting of 12 ppm Oil should be removed. The effluent must be of quality to use as RO system feed.
Which ways may be best for this treatment?
Thank u all.
Taxonomy
- Treatment
- Treatment Methods
- Sewage Treatment
- COD Removal
- Sludge Treatment
- RO Systems
- water treatment
8 Answers
-
Add oxidants, permanganate or hydrogen peroxide. There are off the shelf products that will get your COD back in line. Make sure there is a carbon source, so you can feed the microbes. This will remove/degrade the hydrocarbons also.
Some oxidants have a greater ability than others to remove electrons from other compounds. Oxidants can range from very powerful, capable of oxidizing most compounds with which they come in contact, to rather weak. Both F2 and Cl2 are powerful oxidants: for example, F2 will oxidize H2O in a vigorous, potentially explosive reaction. In contrast, S8 is a rather weak oxidant, and O2 falls somewhere in between. Conversely, reductants vary in their tendency to donate electrons to other compounds. Reductants can also range from very powerful, capable of giving up electrons to almost anything, to weak. The alkali metals are powerful reductants, so they must be kept away from atmospheric oxygen to avoid a potentially hazardous redox reaction.
A combustion reaction, first introduced in Section 3.2 "Determining Empirical and Molecular Formulas", is an oxidation–reduction reaction in which the oxidant is O2. One example of a combustion reaction is the burning of a candle, shown in Figure 3.9 "An Example of a Combustion Reaction". Consider, for example, the combustion of cyclohexane, a typical hydrocarbon, in excess oxygen. The balanced chemical equation for the reaction, with the oxidation state shown for each atom, is as follows:
C 6 −2 H 12 +1 +9O 2 0 →6C +4 O 2 −2 +6H 2 +1 O −2 (3.31) (3.31)C6−2H12+1+9O20→6C+4O2−2+6H2+1O−2
If we compare the oxidation state of each element in the products and the reactants, we see that hydrogen is the only element whose oxidation state does not change; it remains +1. Carbon, however, has an oxidation state of −2 in cyclohexane and +4 in CO2; that is, each carbon atom changes its oxidation state by six electrons during the reaction. Oxygen has an oxidation state of 0 in the reactants, but it gains electrons to have an oxidation state of −2 in CO2 and H2O. Because carbon has been oxidized, cyclohexane is the reductant; because oxygen has been reduced, it is the oxidant. All combustion reactions are therefore oxidation–reduction reactions.
-
You should carry out a jar test to see how much oil you can remove. Normally DAF can’t remove oil to below 10 ppm.
Depends on what kind of COD reminded in your water, biological process normally can reduce COD to 50 ppm. If the influent soluble COD is 50 ppm, most biological process will not work. What you need is physical (membrane filtration) or chemical (zonation) process to achieve the target.
I am assuming your water is the effluent of MF, why don’t feed the RO directly? If the jar test can’t remove oil anymore, then you need a chemical process to oxidize it.
-
Dear Ahmad,
How much is the flow in l/s?
You can use many ways to remove the COD and oil.
I will list the following:
1) Aerobic bioreactor as bioclenarer: it will remove and digest oil and COD and could reach 7 ppm COD (+/- 5 ppm).
2) DAF system: It could remove oil and part of the COD too. If the system does not reach teh the needed parameter, you can compliment it with an ozone dosing to oxidize the COD.
3) Microbubble with microfiltration: It can remove all these parameters (using variable pore modular system instead of membrane filtration). It is strongly recommended. Sometimes the filtration system could be submerged in the micro aeration basin. We can design the system with the needed modules to reach the desired flow.
The micro-filtered water with variable pore technology is strongly recommended before the membrane use.
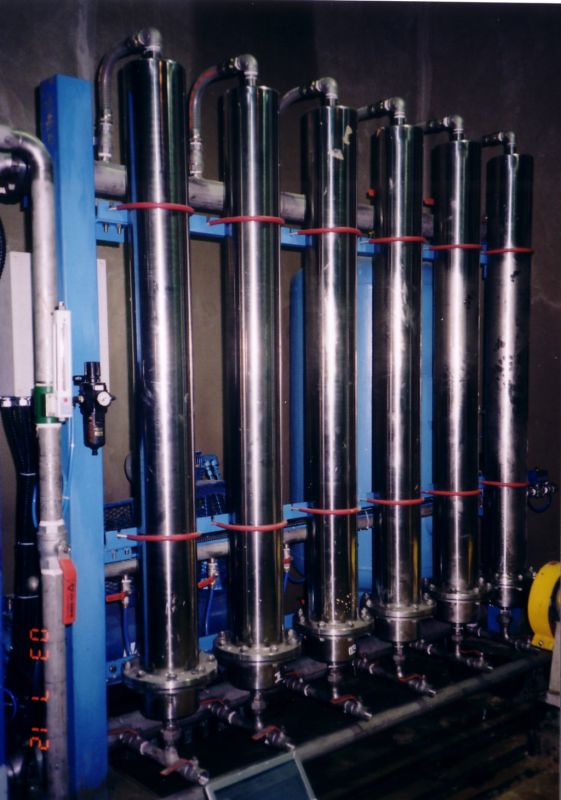
In this following picture you can see a filtration system.
Whatever system must separate the oil in a first step and the remaining COD in a second step.
Regards,
Orlando D. Gutiérrez Coronado
-
Pretreatment such as DAF will help to remove the oil. Then ACF will help to remove the COD.
-
Dear Ahmad,
COD is an important parameter for anaerobic digestion, if the COD is too low the methane production will be low too. it is also important take into account the kind of reactor, the retention time, the temperature. Usually for wastewater the reactors most appropriate are UASB or EGSB, working at low HRT, because the COD of urban wastewater usually is lower than 1000 mg COD/L..Depends on your objective. If you main goal is to recover biogas from the wastewater, the high concentration from 1,000-50,000 mg/L or even higher will be OK. Considering solubility of methane in water (20-30 mg/L), a low concentration is not good for methane recovery. If you focus only on COD removal, low concentration even < 100 mg/L would be OK. The COD is really not important parameter from anaerobic digestion efficiency point of view. The parameters like organic dry matter, N-NH4 content, C:N ratio, HM content play much more important role. a minimum COD is also required at least to produce enough methane to heat the reactor.
The 50 ppm COD is very less hence the Pre-treatment is sufficient.
Mechanically emulsified oil most economically. 12ppp can be removed by activated carbon.
-
Pre treatment would be good for reducing COD and oil parameter
-
Do you know what type of compound is causing the COD? That might change the answer,.
But, not knowing the exact chemistry, I would put an activated carbon bed followed by an ultraviolet TOC destruct unit and a micro-filtration tank ahead of your RO membranes.
I have multiple Ultrapure water trains set up that way at my facility and get very good quality water from the ROs.
Good Luck
-
Electrocoagulation ie passing DC electricity through the water will break down the emulsifying agent and allow the oil to be skimmed off. As hydrogen and oxygen gases are naturally produced during the process, the oil will be lifted to the surface where it can be removed and reclaimed. The emulsifier will sink to the bottom to form a dense sludge that dewaters very easily.