Elevated Lead Levels in Water Supply
Published on by Peter Petersen, Water chemist II (water quality) at Milwaukee Water Works (Retired 2021) in Safety
I understand that lead solubility in water is very low, so how does lead from lead pipes get into the water supply?
Does the lead from the lead pipe react with the carbonates associated with the alkalinity of the water or with the added chlorine as a disinfectant?
If there is any lead (soluble and particulate) in the water source, it is typically removed through the various stages of the water treatment process.
I am therefore curious - where did the lead, e.g. in the Flint incident, come from?
There was a discussion about lead leaching out into the water supply, but where did it come from and why were the lead levels not reduced?
Please explain the lead corrosion chemistry mechanism in simple terms and include any illustrations with references.
Media
Taxonomy
- Water Supply
- Chemistry
- Water Supply & Drainage
- Water Supply Design
- Urban Water Supply
- Pipeline
- Pipeline Installation & Maintenance
7 Answers
-
The chemistry of Lead in water is a very misunderstood science. Most texts immediately assume Lead must be an ionic salt but this is not the fact.
Lead forms complex molecular structures in water and is amphoteric, like Aluminium ion can exist in both cationic and anionic complex ion forms.
When one understands this one can appreciate there are many routes by which lead can enter and remain soluble in potable water.
-
Lead has a low solubility but that is often sufficient to exceed the acceptable standard. This is made worse by low pH and low alkalinity water so that many groundwaters have a low tendency to dissolve lead but surface waters may be much worse. Also the time in which the water is in contact with the lead pipe is a factor. This is why there is often advice to flush systems that have been standing say overnight before use.
One widely practiced technique is to dose phosphate to the water which forms a protective coating on the pipe surface.
One problem is that a lot of testing is done with coupons in tanks of water that does not reflect the pipe situation. That is why a lead pipe test rig is a valuable tool to check the risk of particular water works..
-
The solubility of Lead in water is very much misunderstood. Many basic chemistry texts state that Lead chloride is insoluble but in reality the solubility of PbCl2 is many time greater than the recommended potable water levels. In hypersaline water PbCl2 is highly soluble, this is because lead forms soluble complex anions. Without this capability your car would not run as Pb is soluble in your car battery even though PbSO4 has an order of magnitude lower solubility the PbCl2.
Organic compounds such as acetate, and carboxylates from polymers etc provide even stronger formation coefficient for complex lead anions.
Lead is soluble in potable water is because the ratio of the complex forming anions to lead usually found in potable water is ideal for solubilising small amounts of Lead and it is common practise in water treatment plants to apply additives that enhance the formation of soluble lead complex anions.
-
The Water Network research team has consolidated an answer to the above question.
Further input from members is welcome and appreciated.
Lead does not react with water under normal conditions. Reactivity will increase if lead comes in contact with moist air beforehand.
Lead removal from water:
· An optimized water treatment process should remove particulate lead.
· Oxidized lead is removed as particles.
· Soluble lead is efficiently removed if raw turbidity is present as it sorbs onto particulate material. With more particles before clarification and filtration, more soluble lead will be removed.
Health effects:Lead and its components are generally toxic.
Lead negatively affects both the environment and human health.
Concentrations of lead in plants above 500 ppm negatively affect their growth.
Human overexposure to lead causes colics, skin pigmentation and paralysis. Lead poisoning leads to neurological or teratogenic symptoms.
The human body contains 10-20% of lead and women are more susceptible to lead poisoning, while children and absorb as much as 40%.
Elevated lead levels in children cause lower IQ, behavioral changes and concentration disorder.
If in 10% of samples lead levels exceed 15 ppb (µg/L), according to The Lead and Copper Rule , the water utility must reduce the level of lead.
The Lead and Copper Rule has to be respected due to EPA and WHO standards.
Lead gets in drinking water almost entirely from the distribution system.
Lead gets in the water due to corrosion of pipes (an oxidant reacts with iron, lead, or copper) or from brass and solder fittings.
Depending on water chemistry, lead is present in water in a soluble or insoluble form.
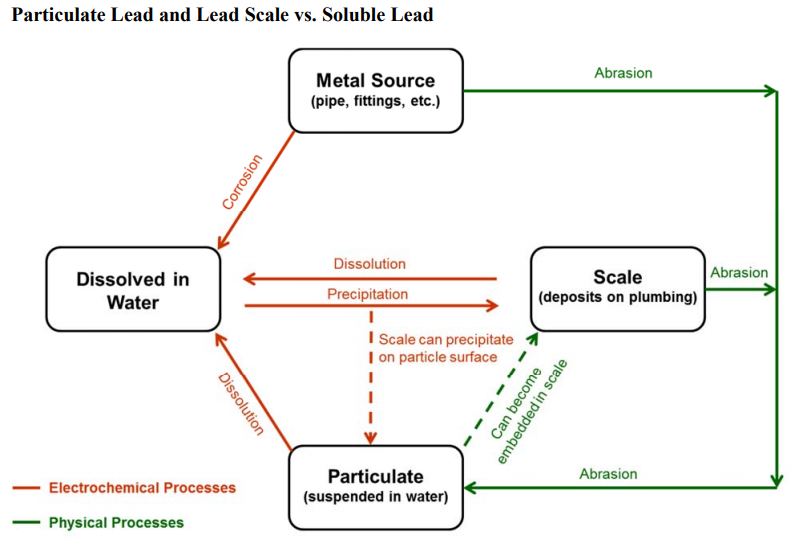
Image: Sources of lead in drinking water
Image source: waterrf.org/PublicReportLibrary/4409.pdf
Soluble lead is dissolved in water.Insoluble lead precipitates onto the pipe surface.
Controlling lead levels in water:
· Adjusting the water chemistry
· High-velocity flushing to remove particulate lead
· Removing materials in the distribution system that contain lead.
Adjusting water chemistry:
When oxidants react with elemental lead, corrosion occurs.
Pipes are protected in water by the passivation layer – a crust on the inside of the pipes.
Orthophosphate is added to water to create this protective layer.
Orthophosphate bonds with lead in the pipes and creates a coating which protects the metal in the pipes from water by preventing the oxidants from reacting with lead.
The coating has a function only if orthophosphate is continuously added to water . Otherwise, the coating will break and oxidants will react with elemental lead and oxidize it.
Oxidnats can be dissolved oxygen in water or chlorine disinfectant.
2Pb(s) + O2(g) + 2H2O(l) -> 2 Pb(OH)2(s)
Dissolved oxygen in water will react with elemental lead and create lead hydroxide.Oxidized lead will not stick to the pipes but dissolve in water and contaminate it.
Chloride and chlorine will additionally enhance the corrosion.
Chlorine is the most common disinfectant. Chloride comes from road salt during winter or as ferric chloride used for disinfection.
When the chloride to sulfate concentrations ratio exceeds 0.58, the lead is much more likely to corrode.
Water pH and hardness:· At pH values below about pH 5, iron, lead, and copper corrode (soluble Pb(II)).
· At pH above 9 these metals are protected.
Higher pH, i.e. higher alkalinity, protects the pipes from corrosion.
Acidic water increases the amount of soluble lead in water as it increases the solubility of lead carbonates which contribute to the protective layer.
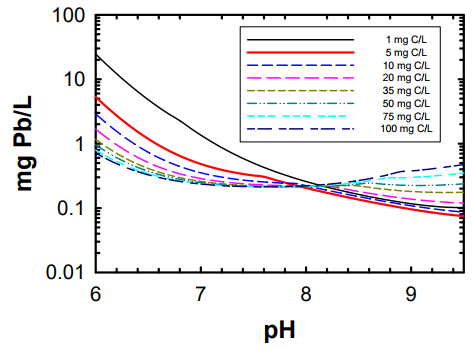
Image: Theoretical impact of pH on lead solubility in drinking water under ideal equilibrium conditions - assumes Pb(II) and no orthophosphate.
Image source: Adapted from Schock and Lytle 2011, waterrf.org/PublicReportLibrary/4409.pdfSoft water enhances corrosion. In hard water, the amount of lead may be decreased as it will bind with carbonates (PbCO3 or Pb(CO3)22-).
Sources:
· http://www.lenntech.com/periodic/water/lead/lead-and-water.htm
· http://cen.acs.org/articles/94/i7/Lead-Ended-Flints-Tap-Water.html
-
EPA and the Centers for Disease Control and Prevention (CDC) agree that there is no known safe level of lead in a child's blood. Lead is harmful to health, especially for children.
Lead can enter drinking water when service pipes that contain lead corrode, especially where the water has high acidity or low mineral content that corrodes pipes and fixtures. The most common problem is with brass or chrome-plated brass faucets and fixtures with lead solder, from which significant amounts of lead can enter into the water, especially hot water.
Homes built before 1986 are more likely to have lead pipes, fixtures and solder. The Safe Drinking Water Act (SDWA) has reduced the maximum allowable lead content -- that is, content that is considered "lead-free" -- to be a weighted average of 0.25 percent calculated across the wetted surfaces of pipes, pipe fittings, plumbing fittings, and fixtures and 0.2 percent for solder and flux.
Corrosion is a dissolving or wearing away of metal caused by a chemical reaction between water and your plumbing. A number of factors are involved in the extent to which lead enters the water, including:
- The chemistry of the water (acidity and alkalinity) and the types and amounts of minerals in the water,
- The amount of lead it comes into contact with.
- The temperature of the water.
- The amount of wear in the pipes,
- How long the water stays in pipes, and
- The presence of protective scales or coatings inside the plumbing materials.
-
These links should provide all the answers that you seek:
http://www.lenntech.com/periodic/water/lead/lead-and-water.htm
http://cen.acs.org/articles/94/i7/Lead-Ended-Flints-Tap-Water.html
http://www.waterrf.org/PublicReportLibrary/4409.pdf (very detailed, PDF)
-
Lead is dissolved in very small amounts by acid water. The short term solution is to add lime to correct the ph and the long term to replace the lead pipes with plastic.