How to reduce TDS level in golf course irrigation water?
Published on by JP Kwon, Castlewood Country Club - Board of Director in Technology
I would like to get some suggestions on improving water quality.
The water is being used for Golf Course irrigation purpose and not for potable water. I am attaching the Water sample analysis results (we have 2 different water sources). We are trying to reduce TDS level to the recommended standards.
How can we best reduce the TDS levels?
Media
Taxonomy
- Irrigation
- Purification
- Quality
- Water Quality
- Water Quality Management
- Water Quality Monitoring
- water treatment
- Green Technology
- Total Dissolved Solids (TDS)
26 Answers
-
The Water Network research team has consolidated an answer to the above question.
Further input from members is welcome and appreciated.Total Dissolved Solids (TDS) are all the minerals, salts, metals and ions (cations and anions) in the water.
Pure water is a universal solvent – it easily dissolves the inorganic salts (Ca, Mg, Na, bicarbonates, chlorides and sulphates) and some of the organic matter, hence the dissolved solids in water.
The biggest concern for irrigation water quality is salinity. Salts can affect the crop yield and soil structure.
Dissolved solids concentration is often called water’s salinity .
Water salinity is, according to Heath, 1983, classified into:
- fresh, 0-1,000 mg/l;
- slightly saline, 1,000-3,000 mg/l;
- moderately saline, 3,000-10,000 mg/l;
- very saline, 10,000-35,000 mg/l;
- briny, more than 35,000 mg/l.
There are two different problems with dissolved salts in water: total salinity and sodium (from sodium chloride).
The soil salinity will increase and affect the plants as saline water used for irrigation evaporates while the dissolved salts remain.
High sodium and low calcium levels of soil or water make the irrigation less efficient as they reduce the water infiltration rate so not enough water reaches the plant and the growth is reduced.
Mild salt effect can, however, go unnoticed especially due to the uniform growth reduction of plants.
Moreover, the irrigation method depends on the total dissolved solids in the water. For example, drip irrigation is suitable for saline water.
The following guidelines for irrigation water have been successfully used in agriculture.
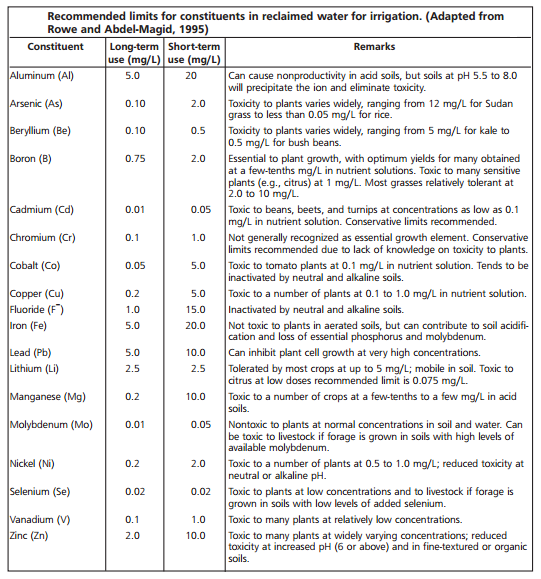
Image: Irrigation water standards
Image source: www.fao.org/docrep/003/T0234E/T0234E01.htmMethods for overcoming the limitations of irrigation water and analytical procedures for the laboratory determinations are given in several publications:
- USDA Handbook 60 (Richards 1954),
- Rhoades and Clark 1978,
- FAO Soils Bulletin 10 (Dewis and Freitas 1970),
- Standard Methods for Examination of Waters and Wastewaters (APHA 1980).
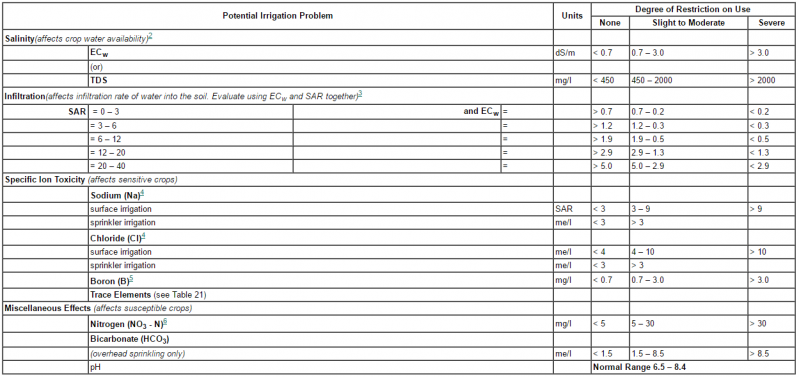
Image: Guidelines for interpretations of water quality for irrigation1 Adapted from University of California Committee of Consultants 1974.
2 ECw means electrical conductivity, a measure of the water salinity, reported in deciSiemens per metre at 25°C (dS/m) or in units millimhos per centimetre (mmho/cm). Both are equiva-lent. TDS means total dissolved solids, reported in milligrams per litre (mg/l).
3 SAR means sodium adsorption ratio. SAR is sometimes reported by the symbol RNa. See Figure1 for the SAR calculation procedure. At a given SAR, infiltration rate increases as watersalinity increases. Evaluate the potential infiltration problem by SAR as modified by ECw.Adapted from Rhoades 1977, and Oster and Schroer 1979.
4 For surface irrigation, most tree crops and woody plants are sensitive to sodium and chlor-ide; use the values shown. Most annual crops are not sensitive; use the salinity tolerance tables (Tables 4 and 5). For chloride tolerance of selected fruit crops, see Table 14. With overhead sprinkler irrigation and low humidity (< 30 percent), sodium and chloride may be absorbed through the leaves of sensitive crops. For crop sensitivity to absorption, see Tables 18, 19 and 20.
5 For boron tolerances, see Tables 16 and 17.
6 NO3 -N means nitrate nitrogen reported in terms of elemental nitrogen (NH4 -N and Organic-N should be included when wastewater is being tested).
Image source:
www.fao.org/docrep/003/T0234E/T0234E01.htmTwo commonly used methods for removing TDS in irrigation water are reverse osmosis and deionization.
Distilation and electrodialisis produce very pure water but also cost a lot and are, therefore, not the best methods for purifying irrigation water.
Reverse Osmosis
Reverse osmosis (RO) is the most cost-effective water purification method for irrigation.
It removes 95-99% od TDS.
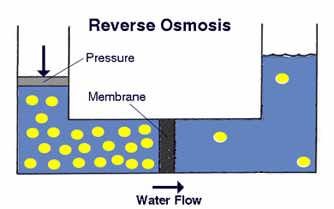
Image: Reverse Osmosis
Image source: www.tdsmeter.com/what-is?id=0013Osmosis is a spontaneous movement of the molecules in the solvent through a semi- permeable membrane. The molecules tend to “ go” in that direction that will equalize the concentrations of the two sides.
Reverse osmosis (RO) is a process in which the particles move in the opposite direction than in natural osmosis. For this process, pressure is needed – the hydrostatic pressure needs to be greater than the osmotic pressure.
The RO membrane has pores large enough to admit water molecules for passage while ions such as Ca2+ and Mg2+ remain behind and are flushed away by excess water into a drain. The resulting soft water supply is free of hardness ions without any other ions being added.
Membranes have a limited capacity, requiring regular replacement.
The degree of removed salts depends on the RO pressure, membrane, TDS and temperature. Efficiency depends on the membrane cleanliness.
Brine water is a byproduct of RO and it needs to be properly disposed.
Deionization
Deionization is very effective and water produced is of better quality than required for irrigation.
The cost of deionization increases with the amount of TDS is water.
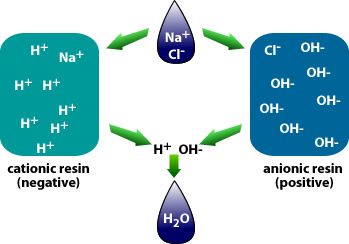
Image: Deionization
Image source: www.tdsmeter.com/what-is?id=0015Deionization ( DI) is a water filtration process where TDS are removed from water through ion exchange by controlling the electric charge of ions in the water.
The process of deionization uses two resins that are opposite in charges – the cationic ( negative) and the anionic ( positive).
DI resins attract non- water ions and replace them with water ions:
The cationic resin will attract the positively charged ions in the water (Ca2+, Mg2+, Na+) and release an equivalent amount of hydrogen (H+) ions.
The anionic resin will attract the negatively charged ions ( HCO3-, Cl-, SO42-) and releases an equivalent amount of hydroxide (OH-).
The hydrogen and hydroxide ions then combine to form water. (H+ + OH- = HOH or H2O.)
Compared to other filtration and purification methods, DI has a relatively short filter cartridge life and once it begins to fail, the TDS level of the purified will “ rise” exponentially.
Costs of RO is generally 5-6 times lower than of deionization.
We, therefore, suggest RO as the best treatment method for irrigation water on a golf course.
Read about the removal of individual salts in Irrigation Water Quality for Greenhouse Production.
Sources:
-
The Water Network research team has consolidated an answer to the above question.
Further input from members is welcome and appreciated.
Total Dissolved Solids (TDS) are all the minerals, salts, metals and ions (cations and anions) in the water.
Pure water is a universal solvent – it easily dissolves the inorganic salts (Ca, Mg, Na, bicarbonates, chlorides and sulphates) and some of the organic matter, hence the dissolved solids in water.TDS in water has different origins - natural sources (leaves, silt, plankton), sewage, urban run-off, industrial wastewater, and chemicals used in the water treatment process.
They also come from rocks and air that contain certain minerals.
Another source of TDS are the metals water picks up while going through pipes while being distributed.TDS is directly related to the purity of water and the quality of water purification systems and affects everything that consumes, lives in, or uses water.
TDS concentration is a secondary drinking water standard.
Therefore, elevated levels are not a health hazard, but the water has greater hardness, may create deposits and be corrosive, water may be coloured and have a salty and brackish taste.
By the EPA standards TDS should not exceed 500 mg/l.
By the WHO standards TDS in water classify as following:Level of TDS [mg/l]
Rating
Less than 300
Excellent
300 - 600
Good
600 - 900
Fair
900 - 1,200
Poor
Above 1,200
Unacceptable
For comparison, it is useful to state that most aquatic ecosystems with different fish fauna tolerate TDS levels of 1000 mg/l.
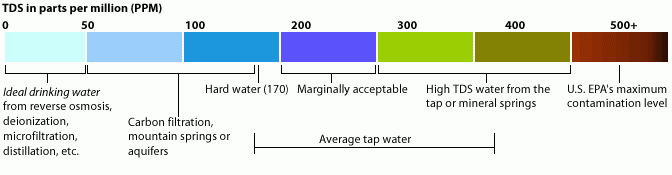
Diagram 1: TDS in ppm
Source: tdsmeter.com/what-is#whatTreating TDS depends on which solids are dissolved in the water:
- If TSD are calcium, magnesium or iron a water softener should be used.
- If concentrations of sodium, chloride, or potassium are elevated reverse osmosis should be used as a water treatment.
- For high to iron, manganese, arsenic concentrations or total hardness in general, other methods should be used.
* TWN Team has written a detailed answer on how to remove As from drinking water. Find it here.
Water softening
Water softening is the removal of metal cations (positively charged ions), such as Ca and Mg, from hard water.
Water softening is usually done by:
Lime softening


Diagram 2: Lime
Source: limemanufacturer.com/hydrated-limes.htmlLime softening relies on adding limewater (a diluted solution of calcium hydroxide) to make the water softer by removing ions by precipitation.
Pure limewater is clear and colorless, with a slight earthy smell and an alkaline bitter taste of calcium hydroxide. The term lime refers to the alkaline mineral, and is unrelated to the acidic fruit.
A saturated solution of lime has a pH of 12,3.As lime in the form of limewater is added to raw water, the pH is raised and the equilibrium of carbonate species in the water is shifted. Dissolved carbon dioxide (CO2) is changed into bicarbonate (HCO3-) and then carbonate (CO32-). This action causes calcium carbonate to precipitate due to exceeding the solubility product.
Additionally, magnesium can be precipitated as magnesium hydroxide in a double displacement reaction.Unlike ion exchange softening, where sodium is exchanged for calcium and magnesium ions, there is a substantial reduction in TDS whereas in ion exchange softening there is no significant change in the level of TDS.
Ion-exchange resins


Diagram 3: Ion-exchange resin
Source: en.wikipedia.org/wiki/Ion-exchange_resinIon exchange is a physical- chemical process in which ions are swapped between a solution phase and solid resin phase.
Ion exchange resins are ion exchange polymers that are insoluble. They form small beads that are porous (water can pass through them). That way they provide a large surface area.
The process is named due to some ions being trapped and others released.
Ion-exchange resins are used to replace the magnesium and calcium ions in hard water with sodium ions.
Fresh resin contains sodium ions at its active sites. When in contact with a solution containing magnesium and calcium ions (but a low concentration of sodium ions), the magnesium and calcium ions preferentially migrate out of solution to the active sites on the resin, being replaced in solution by sodium ions.
This process reaches equilibrium with a much lower concentration of magnesium and calcium ions in solution than was started with.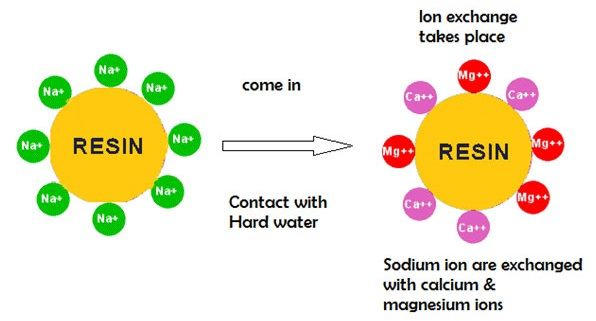
Diagram 4: Ion-exchange
Source: benbellsoftenersystems.in/water-softener/The resin can be recharged by washing it with a solution containing a high concentration of sodium ions (e.g. large amounts of common salt (NaCl) dissolved in it). The calcium and magnesium ions migrate off the resin, being replaced by sodium ions from the solution until a new equilibrium is reached. The salt is used to recharge an ion-exchange resin which itself is used to soften the water.
Reverse osmosis
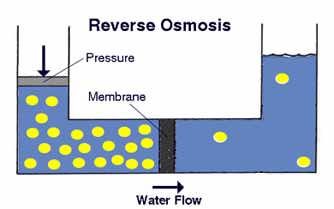

Diagram 5: Reverse Osmosis
Source: tdsmeter.com/what-is?id=0013Osmosis is a spontaneous movement of the molecules in the solvent through a semi-permeable membrane. The molecules tend to “go” in that direction that will equalize the concentrations of the two sides.
Reverse osmosis (RO) is a process in which the particles move in the opposite direction than in natural osmosis. For this process, pressure is needed – the hydrostatic pressure needs to be greater than the osmotic pressure.
The RO membrane has pores large enough to admit water molecules for passage while ions such as Ca2+ and Mg2+ remain behind and are flushed away by excess water into a drain. The resulting soft water supply is free of hardness ions without any other ions being added.
Membranes have a limited capacity, requiring regular replacement.
Carbon filtration
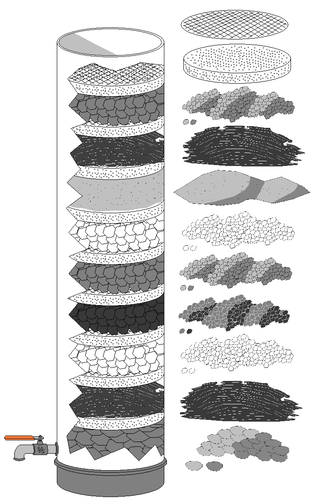

Diagram 6: Activated carbon filtration
Source: en.wikipedia.org/wiki/Carbon_filtering#/media/File:Water_Filtration_Systems.pngCarbon filtering is a method that uses a bed of activated carbon to remove contaminants and impurities, using chemical adsorption (adhesion of atoms, ions, or molecules from a fluid or dissolved solid to a surface).
Activated carbon traps pollutant molecules inside the pore structure of the carbon substrate.
Each particle/granule of carbon provides a large surface area/pore structure, allowing contaminants the maximum possible exposure to the active sites within the filter media.
(One pound of activated carbon contains a surface area of approximately 100 acres.)Because of its molecular makeup, activated carbon can adsorb well, meaning that it can take in or collect many organic molecules on its surface.
Granular activated carbon filters are inexpensive and maintenance involves replacing six to twelve cartridges a year, depending on the quality of the raw water and the filter media.
Active charcoal carbon filters are most effective at removing chlorine, sediment, volatile organic compounds, taste and odor from water. They are not effective at removing minerals, salts, and dissolved inorganic compounds.
Distillation
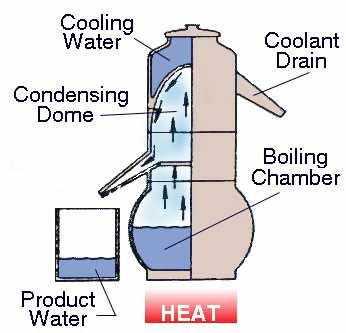

Diagram 7: Distillation
Source: tdsmeter.com/what-is?id=0014Distillation is a procedure by which the liquid is purified by heating and cooling. It is a process of separating the component or substances from a liquid mixture by selective evaporation and condensation.
It is a physical separation process and not a chemical reaction!
Distillation may result in complete separation (nearly pure components), or it may be a partial separation that increases the concentration of selected components of the mixture.
Deionization
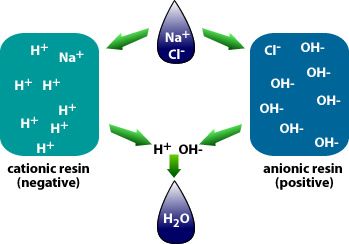

Diagram 8: Deionization
Source: tdsmeter.com/what-is?id=0015Deionization (DI) is a water filtration process where TDS are removed from water through ion exchange by controlling the electric charge of ions in the water.
The process of deionization uses two resins that are opposite in charges – the cationic (negative) and the anionic (positive).
DI resins attract non-water ions and replace them with water ions:
- The cationic resin will attract the positively charged ions in the water (Ca2+, Mg2+, Na+) and release an equivalent amount of hydrogen (H+) ions.
- The anionic resin will attract the negatively charged ions (HCO3-, Cl-, SO42-) and releases an equivalent amount of hydroxide (OH-).
The hydrogen and hydroxide ions then combine to form water. (H+ + OH- = HOH or H2O.)
A single deionization cycle may not remove all the TDS.
Some of the ions will not be attracted by the resins, so running the DI water through a second cycle will provide additional purification.Compared to other filtration and purification methods, DI has a relatively short filter cartridge life and once it begins to fail, the TDS level of the purified will “rise” exponentially.
Additional resources:
Detailed TDS and safe water information
WHO Guidelines for drinking-water quality
EPA drinking water standards and health advisories table
Read more related content on total dissolvde solids ( TDS) here.
Related question: What is the best range for TDS in potable water? -
Apart from high nitrates your water would appear to have potential for a nice potable water (hard yes). Can you say what affect using this water on the grass has or how you are treating it.
-
JP Kwon, I know you posted this some time ago. Are you still having the same issues or have you corrected them already? I noticed your sodium levels are high too. That could cause some issues in maintaining fertile soil. For the sodium you may want to try a remediation product we distribute or one that we manufacture. I can let you know more if your interested. As for the TDS, if your still having issues on this, let me know too. I can give you some sugestions that we have found very useful in the past.
-
Goood point. I think SOCO Polymer is also a solution to reduce water cost.
-
Dear Mr. Kwon,
Can you please share the measure you have been adobpeted for reduction of TDS.
-
My company has a product that processes "dirty" water into distilled water while it is producing electricity. I recommend that you process about 50% of your well water- which will become 55% due to recycling water condensed from our system's operation- and blend the results with your "raw" well water. This should get you to your target while it is also reducing your power requirements- rather than adding load to your current power demands- at a lower cost per kWh than you are likely paying now.
-
Help!!! Anyone have any experience/comment on " in-line catalytic water treatment system" ?? Your candid comment and reply would be highly appreciated.. I got a recommendation to investigate an in-line catalytic water treatment system. I was told that It operates by a combination of the following: 1. High turbulence of the water through a specially designed catalytic chamber. 2. By the creation of a small electrical field around the chamber casing. This combination causes a separation of the mineral particles in the water, which changes their behavior. Particles, which were previously attracted to each other now repel and separate into much smaller individual particles. It eliminates the cohesion that exists between the mineral particles in the water. Thus salt particle size reduction allows the salt ions to pass quicker throughout the soils and not get caught up in the root zone for plants.
-
TDS Reduction
We provide nanofiltration, anything bigger than 11nm gets filtered out. That should answer your problem at a very reasonable cost. You'll probably need two of our big machines, that treat 6,000 liters per hour. That will cost you €25,000 ex-factory Holland (where we manufacture them). No service or maintenance for 5-8 years, energy efficient, check out the website www.thewaterfallproject.com, contact details on there.
-
You have received many correct suggestion about using RO. Nano is not advisable, unless the SAR value of treated water is in range after treatment. Adding anything in water would increase TDS !!!
-
RO is by far the best solution, most common, prooven, easy to operate & mainten. Treat 50% of water through RO and blend 50% untreated water to achieve target values. Alternately Nano Filtration can also be explored which will reduce the operating cost. Since the multi-valent ions are in considerable amount, NF will reduce TDS to desired extent. (however this will be for 100% capacity)
-
Removal of TDS
The most feasible method is to add (Ca(OH)2) to water for irrigation purposes. It precipitates almost all kinds of metal cations and metal anions from water.
-
Since your TDS is low and close to your goal, using the basic RO solution may be the least expensive and most dependable solution in the long run. This would be a low pressure system and the stream could be blended (i.e. you would not have to treat the entire stream).
-
Cleaning this water in batches using the Archaea species of microbes will chelate the toxic metals into their nutrient form and all organic compounds will be reduced into their elemental form. At this point the water will be in a potable state. Filtering then becomes a robing of nutrients. Reuse for the lawns/turfs or put back into the county reservoir. There is a drought going on I have heard.
-
You may find that RO will be the most economical way to address your question. Use small RO to treat part of the stream and blend RO product with the rest of the water to achieve targeted TDS. To increase overall system recovery you can apply brine recovery RO treating primary RO concentrate. What drives to have TDS
-
JP We can do it with a new innovated inorganic Nano coagulant for you.
-
TDS Levels
TDS levels can be reduced by using a lava rock and biofilter combination. The only company I know that has this system is Sho-Me Hydroponics in Nixa, MO.
-
The best solution is Nano filtration as the bivalent ions are remove by Nano filters. We have used this in some of the applications.
-
JP With bag filter you only remove supended solids and not dissolved solids.
-
If you have two water sources . there is not required ro system. because up to 500 tds is ok for irrigation. you can use only softner for hardness of water.or you can blending of two water sources. hardness is harmful for irrigation and plantation.
-
Mr Kwon, beyond doubt, the Hydro Dynamic Magnetic Resonance technology is the answer to your question. It reduces the TDS by breaking the water-macro-molecules into their basic 6-molecule clusters, thus releasing the salt molecules and reclustering these. The result is significant reduction in TDS and as spinn-off easier uptake of the water through the stomata and roots. Check also my Linkedin profile for several posts on the dynamics of this technology that uses flanges with permanent magnets. No power connection needed. After a one-off CAPEx, the next 7-15 years no OPEX for power, filter replacement or any other maintenance. Trust the above makes sense and look forward to communicate one-2-one with you on this option. regards, Jan Willem
-
TDS Reduction
Depending on the chemical characteristics, some components of TDS can be removed by technologies other than RO, the majority do not result in a net reduction in TDS, but serve to exchange ions. If you can provide a detailed water chemistry analysis I may be able to suggest a few new technologies that are of current interest in the oil & gas sector. However, when comparing "equivalent" technologies attempting to address the same market opportunity, in this case TDS removal, generally there is very little difference in cost - particularly where there are few competing technologies. Generally the differentiating factors are rated to operations cost and site specific factors.
-
Unlike reverse osmosis systems, Ultrafiltration does not remove dissolved salts from water. Nano filtration would be cheaper than RO
-
Infotec would be happy to explore with interested parties the opportunities for maximizing the opportunities to increase attenuation allowing grey water to be used more effectively for golf course and sport field watering, whilst enhancing the self cleansing properties of drainage catchments.
-
JP, We deal with these type applications and have a proven technology that will allow for you to effectively use the water for irrigation. For more information please contact me at 4794592254. We would like to discuss how we can help. EW
-
Only way ... http://www.nobel.rs/nobel-proizvodi.php?kateg=2