Consequences of drinking harvested rainwater with lower pH
Published on by Sharon van Tuylen, Hydrologist and IWRM in Technology
We are promoting rainwater harvesting in Guatemala. Water analysis indicated that the harvested rainwater has a lower pH value than what the U.S. Environmental Protection Agency suggests. They say water pH value is a secondary drinking water standard and recommend a pH between 6.5 and 8.5 for drinking water.
The pH of the harvested rainwater in our area varies between 4 and 5.
Are there any negative effects on the human metabolism drinking water with low pH level? Can we slightly increase the pH level by any household water treatment methods?
Taxonomy
- Water
- Water Harvesting
- Rainwater Harvesting
- Drinking Water Treatment
- Households Treatments
- Biological & Chemical Quality
- Quality
- Water Quality
- Water Supply
- Water Harvesting
- Water Supply
- Food & Beverage
- Public Health
48 Answers
-
Low pH is not that dangerous. Aerated soft drinks like cocacola and pepsi have pH even lower than 4. But it shall be a good idea to restore the pH to an acceptable level through simple and natural means and contact with alkaline rocks like limestone which should be easily possible because the harvested rain water is given a pretreatment and stored.
-
drinking acid rain? not a good idea. look at what it does to the metal parts of a car. no bueno. you can easily and cheaply increase it healthily treat that if its for a community. impound in a large pond. put fish and plants. use a limestone filter before water enters pond.
-
drinking acid rain? not a good idea. look at what it does to the metal parts of a car. no bueno. you can easily and cheaply increase it healthily treat that if its for a community. impound in a large pond. put fish and plants. use a limestone filter before water enters pond.
-
Water with low pH has adverse effect on skin and the lining inside. It can also pick up metals from pipes and storage tanks which have adverse effect on metabolic system. The increased risk of cancer in acidic medium is very important. It is advisable that pH of drinking water is raised to fall within the range recommended by US and WHO. Oxides of calcium and magnesium and soda ash are suitable for raising pH of water.
-
onjour
Bien entendu que nous pouvons augmenter le ph. Mais est ce nécessaire?
La nature nous fournit une eau potable qui a peut être des centaines d'années de formation. Cette eau contient des éléments que seule la nature peut nous apporter. Modifier un facteur de sa composition interférera automatiquement par un déséquilibre sur les autres facteurs. Car la nature à l'avantage sur le scientifique, c'est qu'elle pratique toutes ses actions par petites touches. L'homme ne sait que déséquilibrer ce que la nature fait
Hello of course that we can increase the ph. but is it necessary?
Nature provides us with drinking water that may be hundreds of years of training. This water contains elements that only nature can bring us. Change a composition factor will interfere automatically by an imbalance on the other factors. Because the advantage on the scientific nature, is that she practices all his actions by small keys. The man knows that unbalance what nature does -
Just to add something to health effects discussion.
There is plenty of research showing the link between pH and cancer. Cancer thrives in acidic environment, and doesn't survive in a normal, more alkaline environment. Cancer cells make body even more acidic as they produce lactic acid.
Actually, too much acidity is an underlying factor in many degenerative diseases -- diabetes, arthritis, fibromyalgia and more.
details available at link
http://www.cancerfightingstrategies.com/ph-and-cancer.html
-
the quality&Personality of water energetic power are destroyed by the filtering challenges.if Global all Research Center's will be silent ,serious i will exposed the same issue i will developed the crises issue treatment & water quality,personality qualification qualified through my process i will created this water of all Global 100% pure & energetic.in one liter X 1000Gram X 1000000Numbers of healthy germs energetic ,vitamins= 1000000000numers of healthy germs illness germ free 10% i one of the Global one Person created this impossible work. so arranged a meeting with me on my place i will exposed after some Negotiation taken over some exchanged Dollar's & gift the life from my hand the great development of this Global like as a miracle touched & 24hours Sun rise in this Global Back Bone never set sun never set.
-
the quality&Personality of water energetic power are destroyed by the filtering challenges.if Global all Research Center's will be silent ,serious i will exposed the same issue i will developed the crises issue treatment & water quality,personality qualification qualified through my process i will created this water of all Global 100% pure & energetic.in one liter X 1000Gram X 1000000Numbers of healthy germs energetic ,vitamins= 1000000000numers of healthy germs illness germ free 10% i one of the Global one Person created this impossible work. so arranged a meeting with me on my place i will exposed after some Negotiation taken over some exchanged Dollar's & gift the life from my hand the great development of this Global like as a miracle touched & 24hours Sun rise in this Global Back Bone never set sun never set.
-
The Water Network research team has consolidated an answer to the above question.
Further input from members is welcome and appreciated.
What is pH in drinking water?
The pH level of your drinking water reflects how acidic it is. pH stands for “potential of hydrogen,” referring to the amount of hydrogen found in a substance (in this case, water). pH is measured on a scale that runs from 0 to 14. Seven is neutral, meaning there is a balance between acid and alkalinity. A measurement below 7 means acid is present and a measurement above 7 is basic (or alkaline).
What are the health effects of pH?
The U.S. Environmental Protection Agency (EPA) does not regulate the pH level in drinking water. It is classified as a secondary drinking water contaminant whose impact is considered aesthetic. However, the EPA recommends that public water systems maintain pH levels of between 6.5 and 8.5, a good guide for individual well owners. Water with a low pH can be acidic, naturally soft and corrosive.
Acidic water can leach metals from pipes and fixtures, such as copper, lead and zinc. It can also damage metal pipes and cause aesthetic problems, such as a metallic or sour taste, laundry staining or blue-green stains in sinks and drains. Water with a low pH may contain metals in addition to the before-mentioned copper, lead and zinc.
Drinking water with a pH level above 8.5 indicates that a high level of alkalinity minerals are present. High alkalinity does not pose a health risk, but can cause aesthetic problems, such as an alkali taste to the water that makes coffee taste bitter; scale build-up in plumbing; and lowered efficiency of electric water heaters.
But some research site says that:
People have a higher tolerance for pH levels ( drinkable levels range from 4-11 with minimal gastrointestinal irritation), there are still concerns. pH higher than 11 and lower than 4 causes skin and eye irritation. pH bellow 2.5 causes irreversible skin damage and organ linings.
pH adjustment systems:
There are two primary types of system design for pH adjustments – continuous and batch.
Continuous flow
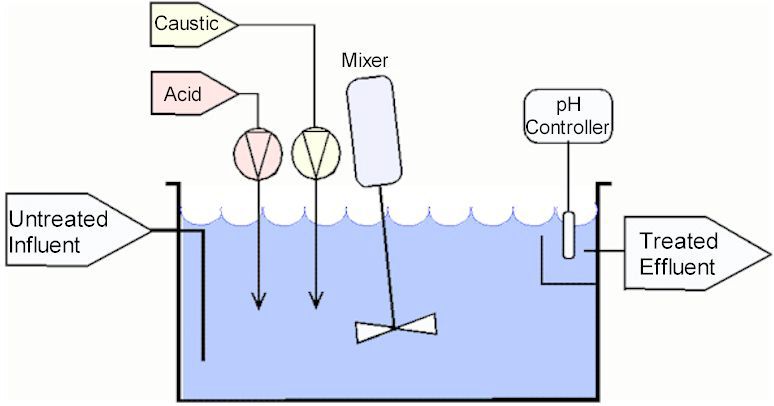 Diagram 1: Continuous flow system.
Diagram 1: Continuous flow system.
Source: phadjustment. comThe tank is constantly full – the amount of influent entering it equal to the treated effluent exiting the tank.
The advantage of this system is that can handle relatively high flows. However, it is not certain that the effluent will always be in range.
Batch
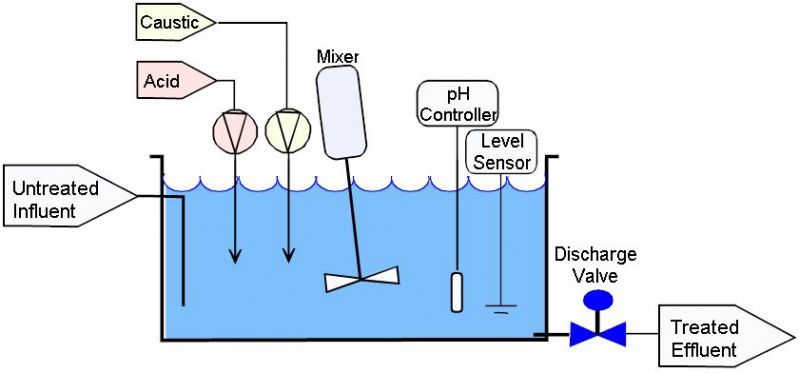 Diagram 2: Batch adjustment system
Diagram 2: Batch adjustment system
Source: phadjustment. comThe batch has a fixed water volume, which is discharged only after fulfilling the criteria.
The influent enters the tank anywhere convenient and exits due to gravity near the bottom, where the port is located.
The batch volume is treated in one cycle.
** The systems shown here are simplified.
pH adjusting methods:
Raising the pH
Lowering the pH
Neutralizing filters
Acid injections
MgO beads
CO2
Soda ash/ sodium hydroxide injections
Neutralizing filters
Neutralizing filters are used if drinking water is acidic.
The pH is increased by the addition of the neutralizing material.
It is important to highlight that the water hardness may increase.
(Water hardness is the amount of dissolved calcium and magnesium in the water - dissolved bicarbonate minerals - calcium bicarbonate and magnesium bicarbonate.)Neutralizing filters are point-of-entry devices.
Water with pH greater than 6 is treated with calcium carbonate ( limestone) and water with the pH below 6 is treated with the synthetic magnesium oxide.
Untreated water passes through a filter filled with either calcium carbonate or a synthetic magnesium oxide medium and the material dissolves in the water therefore raising the pH level.
The flow rate should not the greater than 2 l/s·m2. The bed should be deep enough to provide sufficient contact time.
The material in the neutralizing filter need refilling and regular backwaching.
If cartridge filters, that retain solids from passing through, are installed before the neutralizing filters, the neutralizing filters will last longer.
After the neutralizing filter a water softener can be added to regulate the water hardness.
The neutralizing filter may result in pressure loss, since the water passes through the finely ground neutralizing material.
The corrosion of the pressure tank and the well pump may occur since the neutralizing filters are installed after the pressure tank.
In case of a high flow rate, liquid injection systems are a better solution.
Magnesium oxide beads in combination
Prill MgO beads are used when the water pH needs to be rasied.
They should be used after reverse osmosis.
Osmosis is a spontaneous movement of the molecules in the solvent through a semi- permeable membrane. The molecules tend to “ go” to the in that direction that will equalize the concentrations of the two sides. Reverse osmosis is a process in which the particles move in the opposite direction than in natural osmosis. The contaminated fluid passes through the membrane and the suspended particles are separated from the liquid. For this process, pressure is needed – the hydrostatic pressure needs to be greater than the osmotic pressure.
Prilly Pure Water Beads raise and balance pH levels of the water to 8,7 without any chemicals.
The beads are made from magnesium oxide which is produced from naturally occurring salts of magnesium found in rich brine deposits located approximately 2, 500 feet below ground. The resulting magnesium oxide is ‘ prilled’ into small, hard pellets by a high temperature firing process which turns them into small ceramic-like pellets.
In addition to adjusting the pH, the beads lower the surface tension of water, remove toxins and pull out heavy metals from water.
Prilly Pure Water Beads last forever and never need to be replaced.
Injection systems:
I Soda ash/sodium hydroxide injection
Soda ash/ sodium hydroxide injections are used if the water is acidic.
When injected into a water system, soda ash (sodium carbonate) and sodium hydroxide raise the pH of water.
Injection systems are a point-of-entry system.
Soda ash or sodium hydroxide solution are injected in the water by a corrosion- resistant chemical feed pump.
The injections are installed before the pressure tank so that the tank ant plumbing systems are protected from corrosion.
Dual treatment is used if the water needs to be disinfected, in addition to being neutralized. A chlorine solution is added with the neutralizing chemical.
With the injection systems water with low pH can be effectively treated – as low as 4.
The chemical storage tanks need to be refilled occasionally.
II Acid injection
Acid injection is used for water with a high pH.
Water with a higher pH can have a soda- like taste that is eliminated with this treatment and the chlorination is improved.
Acid injection is a point-of- entry system.
Acid injection reduces pipe corrosion, since water with the pH above 9 corrodes brass, copper, zinc, aluminum and iron.
A solution of acetic acid is injected into water. Usually white vinegar is used, as it is the cheapest, but citric acid and alum are also an option, as well as more hazardous weak solutions of hydrochloric acid or sulfuric acid if the pH is above 11.
The chemicals need to be refilled occasionally, while wearing the protective goggles, gloves and clothing.
Carbon dioxide
Carbon dioxide is used to reduce pH in alkaline water.
Carbon dioxide, CO2, is a colorless and odorless gas. It is a chemical compound composed of a carbon atom covalently double bonded to two oxygen atoms.
It is used as a pretreatment and sulfuric acid is added in the second step. The main purpose of this secondary acidification is to reduce the bicarbonate content and avoid calcium carbonate precipitation.
Carbon dioxide does not corrode the pipes and the equipment.
It was gives better control of pH than sulfuric acid. It shows self- buffering when reaching neutral pH levels. The self-buffering enables precise end-point control eliminating the danger of lowering the pH too much.
It can be utilized via a completely automated system.
Documents on TWN about pH and drinking water standards:
- WHO guidelines for drinking- water quality
- WHO pH in drinking-water
- EPA drinking water standards and health advisories table
- pH Control in WTP by the Addition of CO2
- Drinking Water Treatment - pH Adjustment
- pH Requirements of Freshwater Aquatic Life
- What is pH and How is it Measured?
- pH Theory and Practice
- The Theory of pH Measurement
-
You might want to doublecheck your water analyzing procedures.
Rainwater should have a pH of 5.6 to 5.8, simply due to the presence of carbonic acid (H2CO3) from the atmosphere.
If the ph is between 4-5 units, that is indicative of a problem. The rainwater may be contaminated. The uric acid levels in bird droppings is high; reaching pH levels of somewhere between 3 to 4.5 – quite acidic.
Rainwater should have relatively low buffering capacity so that it should not take much of any alkaline chemical to raise the pH. Soda ash is an inexpensive and relatively harmless chemical for this purpose.
The water should also be disinfected.
Before treating the water, you should investigate further to determine the source of the contamination.
-
The simple answer is the addition of calcite filters to raise the PH. These are available in backwash models, but they also make upflow models with no back wash needed. Calcite is a very inexpensive material and readily available.
-
Parke
In the USA there are many people living on Rainwater. It depends on the climate and the size of the tank. The tank costs can be inhibitive, but there are many choices.
-
I do not know where your info is coming from but the more pure the water, the better, regardless of P.H. Value! Rain Water is about as pure as it gets, but I'm also unaware of ANYPLACE on the Globe where you can count on Rain Water as your sole supplier without mass storage/storage tanks which are cost prohibitive if even available in remote areas.
Other Technologies you can successfully implement affordably along with your Rainwater Harvesting are: Solar Distillation Panels (can purify almost any kind of contaminated water, have been used to recycle Frac Water, also serve double duty & capture Rain Water), AWG = Atmospheric Water Generators, they capture & create pure water from the moisture in the air & are proven everywhere on the Globe, even in Deserts!
For more info or to begin a dialogue on this topic, please feel free to contact us;
Geosource Foundation in Malibu, California USA
(805) 410-2372
You may also want to see;
-
my friend,you are the best & innocent of one out of Global population 10000Million person/public/voters. an each & every knowledge offer stomach consumption.i.e.food.feeds, water, cold drinks, hot drinks, dishes various tested, tongue tasted like great amazing fish fry, chicken fry, rice with chicken, etc. so in short i am feeling about orphanage of this Global one who are breathing with us all stomach challenging food,feed, water, dishes, etc. so i want the Exchanges of my Knowledge.i have one person out of Global 10000Million people's only one who know the knowledge of 100More issue challenging to all Global person but all are unable to reply the same above matter no use of the mind, heart, mental, knowledge. so i offer myself as a exchanged equal to my knowledge values.
-
Agreed with John Cody.
Lower pH values indicate acidic properties of the water which is not considered as " SAFE DRINKING WATER".
Prof. Dr. Niaz Memon
-
@
Sharon van Tuylen
Potential adverse health reactions related to acidic water are related to the potential for high metal concentrations. This can be a major issue in older distribution systems where lead pipes and fittings may have been used, as plumbo solvency can cause harmful concentrations of lead in the water. Such low pH values may also strip silver nitrate ions form any household filters, reducing their effectiveness in removing bacteriological pathogens and reducing their effective life. High silver concentrations in water can have chronic negative health impacts, but I think this is unlikely to be an issue, as the doses would be low. Silver toxicity is readily identified in any case-it turns the skin and hair blue.
At the pH values of 4-5 the carbonate equilibrium will be shifted towards carbonic acid. However I would be concerned that you are getting such a high degree of acidification in the rain, which would indicate some form of atmospheric pollution. If you are in an urban environment you could be getting dry deposition of nitrous oxides (traffic fumes) or there may be a source of sulphur dioxide. Or there could be a build up of organics in your system, with the acid coming from microbial or algal sources.
I would suggest that you check the water for inorganic nitrogen before supply as nitrates can cause health problems-methemoglonemia. This is often associated with vitamin C micro nutrient deficiencies, and can be fatal to children, especially those fed with formula. You would need to check for both nitrates and nitrites-again the equilibrium of these species is pH dependent.
On the positive side you will get very little microbial activity at such low pH, so the water is likely carry low acute risks associated with bacterial and potentially viral pathogens.
In terms of increasing raising the pH, the simplest solution would be to increase retention time in your systems. Carbon dioxide will dissolve into the water, pushing the carbonate equilibrium towards the carbonate end of the spectrum, providing alkalinity and bringing the pH up towards the more normal range of 6.5 to 8.5. If this process is to slow you could try cascade aeration.
Hope this helps
John Cody
-
It is healthy to drink alkaline water than acidic water. When the pH of water being process for drinking is low, it is advisable to adjust the pH towards alkaline by adding base and run the household filter.
-
A lot of good answers here; I concur that drinking water with a pH below 6.5 is probably not the best. Low pH is also an attribute of distilled and reverse osmosis water, and these water sources are not considered harmful, however.
If the rainwater is purified with ozone, then the oxygen molecules that are released in that process can combine with some of the free hydrogen ions (pH is a measure of free hydrogen ions, a lower number means more are present) which makes more pure water. Another good way to buffer the low pH is to store the rain in a concrete tank rather than plastic or metal. Calcium or sodium bicarbonate are good buffers, as well.
Just plain aeration, that is - introducing a flow of air bubbles into the water like an aquarium, will also help.
It might be worthwhile to identify the element that is causing the acidity. It could be carbon (carbolic acid), nitrogen, (nitric acid), or sulfur (sulfuric or muriatic acid). An analysis would help.
-
EPA secondary standards for drinking water pertain to contaminants that are not considered health threatening but can have undesirable effects. See https://www.epa.gov/dwstandardsregulations/secondary-drinking-water-standards-guidance-nuisance-chemicals, which says that low pH can cause a bitter metallic taste and corrosion of metal water pipes and fixtures. Other sources show that many soft drinks have pH in the range of your collected rainwater, and the acidity of orange juice is even lower.
-
Dear Sharon Van
pH is an indicator of the acid or alkaline condition of water. the pH value is a good indicator of whether water is hard or soft. The pH of pure water is 7. The pH is mostly a result of natural geological conditions at the site and the type of minerals found in the local rock. The pH can also be affected by acid rain. Water with a pH value less than 7 is acidic and tends to be corrosive. In general, water with a pH
While the ideal pH level of drinking water should be between 6-8.5, the human body maintains pH equilibrium on a constant basis and will not be affected by water consumption. For example, our stomachs have a naturally low pH level of 2 which is a beneficial acidity that helps us with food digestion.
Acidic water (low pH) can leach metals from plumbing systems, which can cause pipes to leak. Metals that leach from the pipes (lead from lead pipes or copper from copper pipes) may also cause health problems. Water with a value greater than 7 indicates alkalinity and tends to affect the taste of the water.
A neutralizing filter is used if drinking water is acidic (low pH). It is a simple treatment device that raises the pH of water by adding a neutralizing material. However, it should be noted that the neutralization process may increase water hardness
Soda ash/sodium hydroxide injection for low pH
This treatment method is used if water is acidic (low pH). Soda ash (sodium carbonate) and sodium hydroxide raise the pH of water to near neutral when injected into a water system. Unlike neutralizing filters, they do not cause hardness problems in treated water.
Acid injection for low pH
Acid injection treats water with a high pH by lowering the pH of water to around 7, which eliminates the soda taste and can improve the effectiveness of chlorination
-
I still concur with other readers saying
low pH is not good for human health. I suggest you try and assess the originality of the problem. Is it because of acid rain, is because of chemicals such as paintings from the roof or is the container or tank used for water harvesting. I think you need to do risk assessment if the cause is not natural. Water harvesting is a very good initiative and must be encouraged.
-
Any water that the PH is lower with this values is not good to the human system.Y you are adding more acidity to your system.I would advice since the pH is low try increasing the pH value with household alkaline method.
-
Hello,
Drinking water with a pH lower than 5.00 is not good for health. You have to correct pH to 6.5 minimum vith calcium carbonate or limestone, and measure the pH value after corrction, so it is always under 8.5. You also have to filter this water and to ensure yourself it is desinfected.
-
Yes.... Drinking Water That has a PH Of 5 or less is harmful. It can make Acid reflexes in your systems.You can correct the PH By mineralising(Calcium,Magnesuim, Potasuim) the Water this can Be done By passing your water Thru the Mineral cartridge.This can also be done by adding Herbs and spices to water.
Vishwas Shinde
Mob no 9892126970
-
the lower pH may be caused by acid rain. You need to check the quality of the water you harvested.
-
You must consider how you will sterilise the water, and for the sterilization to be effective you will need to increase the pH, unless it is a UV system you are using, even so at a pH of 4-5 skin irritation, possible irritation of the throat and eyes may also occur, you may consider filtration through a calcite media to increase the pH, but if this water is collected from a roof our surface it should be sterilised.
-
If you collect rainwater properly it is potable right out of the tank. Use plastic piping, non caustic glues, a potable tank, throw a limestone in the tank, monitor regularly and your set.
As mentioned there are several ways to raise the ph and charcoal and UV to filter if you have the concern.
-
Rain water harvesting is really worthy and lucrative for mankind. Very frank, artificially or chemically improving pH value water is not at all good for human being. Sure it creates much more problems for all consumers. Instead of drinking, we can go or utilize it for irrigation purpose.
-
Rainwater is delicious! It makes your skin feel lovely and makes the best cup of tea! We only have rainwater for all our water needs. You only need a fraction of the soaps and washing powders etc as it lathers really well. I'm pretty sure you will get all the electrolytes and salts you need from your food if you have a healthy diet. We don't filter or treat our water at all and we have young children. The biggest concern is definitely your pipes and cooking equipment. If you have metals in your plumbing they could leach into the water at that pH.
1 Comment
-
you may have a disease after decades of drinking rainwater without any treatment. Long term effects need time. Until now, only some of groundwater can be drunk directly without treatment.
-
-
You will not have any issues drinking rain water. Your biggest concern will be bacteria and a small amount of household bleach will solve that and by default raise the PH.
-
For gravity feed this can be done using a sand filter having mixed limestone or corals to adjust it from local quarries a thought for low-tech, low pH is why the lead poisoning happened in Flint, MI, it came out of their piping.
-
You can easily run the low pH water through your household water treatment to bring back the pH. The concern of low pH water is more related to you pipping system, containers. Simple run this water through activated carbon filter will also help to bring water back to close to neutral.
-
Rainwater with a pH of 4 to 5 IS dangerous to health if it is in contact with aluminium metal. Although short-term effects are unlikely to cause problems, the long term effect of drinking acidic water that has been in contact with aluminium can be severe. It is one of the environmental factors that are responsible for the development of Alzheimer's Disease, by stimulating the amyloid cascade process that leads to plaque development in the brain. The risk of AD doubles i areas where the water supply contains only 0.1mg/l of Al. I experienced this problem at first hand in Britain thirty years ago, and was involved in collecting brain samples for analysis later by Chris Exley at Keele University, UK. A number of people almost certainly died with early-onset dementia as the consequence of their exposure. In that case the Al concentration was far above anything you are likely to experience in practical terms, but any action that solubilises aluminium, by allowing water with a low pH to come into contact with it, rendering it bioavaiable, should be avoided at all costs. So by all means collect the water - but avoid allowing it to have any contact with aluminium pans etc, unless the pH has first been raised above 6, at which level aluminium is virtually insoluble.
1 Comment
-
Interesting reminder for me as I recall back in the 1980's working with Leeds University hospital renal unit, looking at the use of Reverse Osmosis and ion-exchange system, to ensure Al levels could be met for water used in renal dialysis wards, high risk patients. Connection of low level of Al and Alzheimer's Disease had been established.
-
-
Hi Sharon,
While I'm not a doctor, I think drinking acidic water is bad for your bones, and digestive system. I would research further before proceeding.
My assumption is that this project is to assist with lower socio-economic development areas?
If this is the case, I would recommend sea shells as a media for the water to pass through to help stabalise the pH, if you are on a shoe string budget. Obviously these will need to be steralised first.
Generally, Caustic soda is dosed in water treatment, to raise pH. Obviously this requires an effective pH monitoring system, linked to a variable speed dosing pump. I recommend you investigate the alkalinity of the water too, as this may be the cause. maybe look at the material the water is collecting from too, the pH you have quoted seems pretty extreme for rainwater. Acid rain? Generally this is produced in larger populated areas.
Hope that helps,
Kind regards,
Shane Blake
1 Comment
-
Acid is a huge problem in Africa. high sugar consumption. All disease is acid by nature. Essential we maintain a balance PH
-
-
Considering that the stomach pH is pretty low, I do not think there would be any harm to the GI tract from drinking the rainwater due to low pH. However, most minerals are more soluble at lower pH - so any container in with which the water comes into contact, may leach more.
-
With such a low conductivity there is no buffering capacity to the water and a stable pH value cannot be tested. There are no health related issues consuming low pH...other than anecdotal stories from non professionals. If you wish to add minerals and test pH, calcite media is inexpensive and easy.
Alfred J. Lipshultz, President
-
Issues could be irritation and scavenging of minerals in the digestive system. More serious is its corrosive effect on metal pipes, storage thanks or other metal components that could come in contact with this water. If there are heavy metals present. They will be leached into the water. Heavy metals have very serious health consequences in humans.
To increase the pH, If using calcium or sodium hypochlorite to disinfect this water, then the pH should go up to range. If you are using a non chemical disinfecting method, then the use of soda ash is recommended.
Most important is to determine what is causing the low pH, most cases is CO2, but it could be due to acid rain. Understanding the make up of this water is important to develop a treatment plan that is economical and effective.
There are plenty of technologies out that could help you solve this challenge.1 Comment
-
First plan of action must be to determine the contributing factor for the low pH.
-
-
The indicator for acidity, alkalinity, or basic is known as the pH value. A pH value of 7 means a substance is neutral. The lower value indicates acidity, and a higher value is a sign of alkalinity. To better understand the range in pH, take a look at these examples:
- Apple Juice - 3 Orange Juice - 3.5 Coffee - 5.5 Milk - 6.2 Baking Soda - 8.5
- Soapy water - 10 Bleach - 12
in our highest quality waters in Alaska (25,000 years old) we have a high pH keeping it alkaline. This is preferable for human health, but it is a 'fine' water and next to the best bottled water in the world.
want more? Just ask Ric Davidge, The Water Czar/Alaska
-
In my opinion the low pH is a problem. This is supported by a pretty good body of research. In addition problem is the fact that rain water contains no minerals. It's been shown that drinking distilled water, wh
-
In Bermuda, the inhabitants utilize limestone roofing which is used to collect rainwater and then conveyed to underground storage tanks.
Aquatic organisms is the major concern with water storage and treatment. If the water has been tested and found to be safe for drinking with the exception of pH, it would be no worse than drinking carbonated soft drinks or coffee; which have low pH values.
-
acid rain. could have lots of sulphur. drinking in huge quantities, that may give you the runs.
the water can be easily treated. run it through a slow sand filter. the microbes in the sand will help with alkalizing the water.
-
Rain water has few if any minerals and hence very little buffer capacity.
to determine normal rainwater pH
First, we calculate the amount of CO 2 dissolved in water under an atmosphere of pressure from Henry’s Law

Since CO 2 makes up 0.0355% of the atmosphere (on the average) and
K CO 2 =2x10-3

Since is in equilibrium with H 2 CO 3 (aq), the first acid equilibrium is normally given by

is predominant. Also since
CO 2 (aq) + H 2 O « H + (aq) + HCO 3- (aq)
The proton and bicarbonate concentrations are equal. Thus

When we substitute the carbon dioxide concentration, and solve for pH, we get
p H = 5.65
Since rain is in equilibrium with the atmosphere, this is the pH expected for natural rain. It is also the pH expected if the body of water is in equilibrium with the atmosphere, and does not contact limestone (e.g., CaCO 3). Adding some limestone to your water tank should increase the pH overtime and restore proper balance.
Yours is a bit lower than 5.65 so it may contain some sulfur compounds forming a bit of sufluric acid but shouldn't be harmful.
1 Comment
-
Drinking treated rainwater might be the only solution for several parts of India in the coming years. Some of the traditional harvesting systems in the rural Rajasthan and Gujarat have worked very well. the "tankas" collect rainwater from rooftops and channels that are painted with limestone slurry. Water passes through a thick layer of sand before percolating into stone lined wells. Water stored in such tanks stays potable and surprisingly tasty.
-
-
Hi Sharon: Rainwater reuse as drinking water would be OK but the water should be modified by adding electrolytes since it will typically be electrolyte poor. You should be able to add this to the water by a simple injector or by putting the collected water into a reservoir holding tank and adding electrolytes manually. Drinking water with a low electrolyte concentration for a long period or time i.e., as the primary supply source would be bad for health. By the way adding the electrolytes will also adjust the pH to within the range of the recommended US EPA range. Really only end trace amounts of micronutrients and overall pH can be adjusted using calcium carbonate. Hope that helps.
-
My research tells me their are negative health effects, this article speaks to the subject http://worldacidrainsecrets.blogspot.com/2012/02/acidic-drinking-water-its-health-risks.html
Another important issues is the leaching effect of acidic water, acidic water has corrosive properties, it leaches substances from everything its stored in and flows thru, plastic storage containers and PVC pipes are all made of toxic materials.
Yes, their are household treatment methods to increase pH, but of equal importance is complete purification. www.truestspring.com nevin@truestspring.com
-
One suggestion it to use limestone I the tank to fair values. Monitoring is still necessary.
https://rainwaterharvesting.wordpress.com/2010/08/28/on-acid-rain-in-india-and-rainwater-harvesting/
-
my friend,you are the best. i want to returned your all struggle effort ,grind by nature produced in the molecule as a Perspiration ,i want to exchanged your perspiration in to dollar's ,this is a happy news for you all.
-
It may be better to let first showers not be collected for a few minutes as these will have maximum acidity. For balance rain water, you may need to add a few minerals/ sodium/ potassium/calcium salts in traces as they do mineralisation of RO water..to make Ph as well as mineral salt content better for water to taste good as pure RO water without minerals is also bitter. Must also check Rainwater for any microbes that float in air and come down with rain.
-
Low pH of the rain water is usually caused by air pollution. We had the same problems in the 80s and 90s of the last century here in Germany. We called it "acid rain". It can destroy trees and whole forests. The reasons are sulphur oxides and nitrogen oxides from traffic, industries and power plants who don´t have sufficient exhaust gas treatment plants. The gaseous pollutants react with the humidity in the air to sulphurous acid and nitrous acid which leads to a low pH in the rain water. The low pH in the rain water can be adjusted by using Calcium Carbonate or Dolomite filters. They remineralize the rain water with Calcium and / or Magnesium and increase the pH at the same time. But you should be aware that the sulfate and sulfite as well as nitrate and nitrite anions are still remaining in the water. Especially nitrite is toxic and for nitrate there are limit values given for the use as drinking water. So please make water analysis before you use this water as potable water. If there is nitrite in the water you cannot drink it. I don´t know the limit value for nitrate in drinking water in your country. Here in Germany the limit value is 50 mg/l. Lots of experts think that this value is still too high and should be lowered.